Influence of Inductive Effect in Organic Residuals Content in IZO Thin Films and the Performance on the Behavior of MIS Capacitors on Plastic †
Abstract
:1. Introduction
2. Materials and Methods
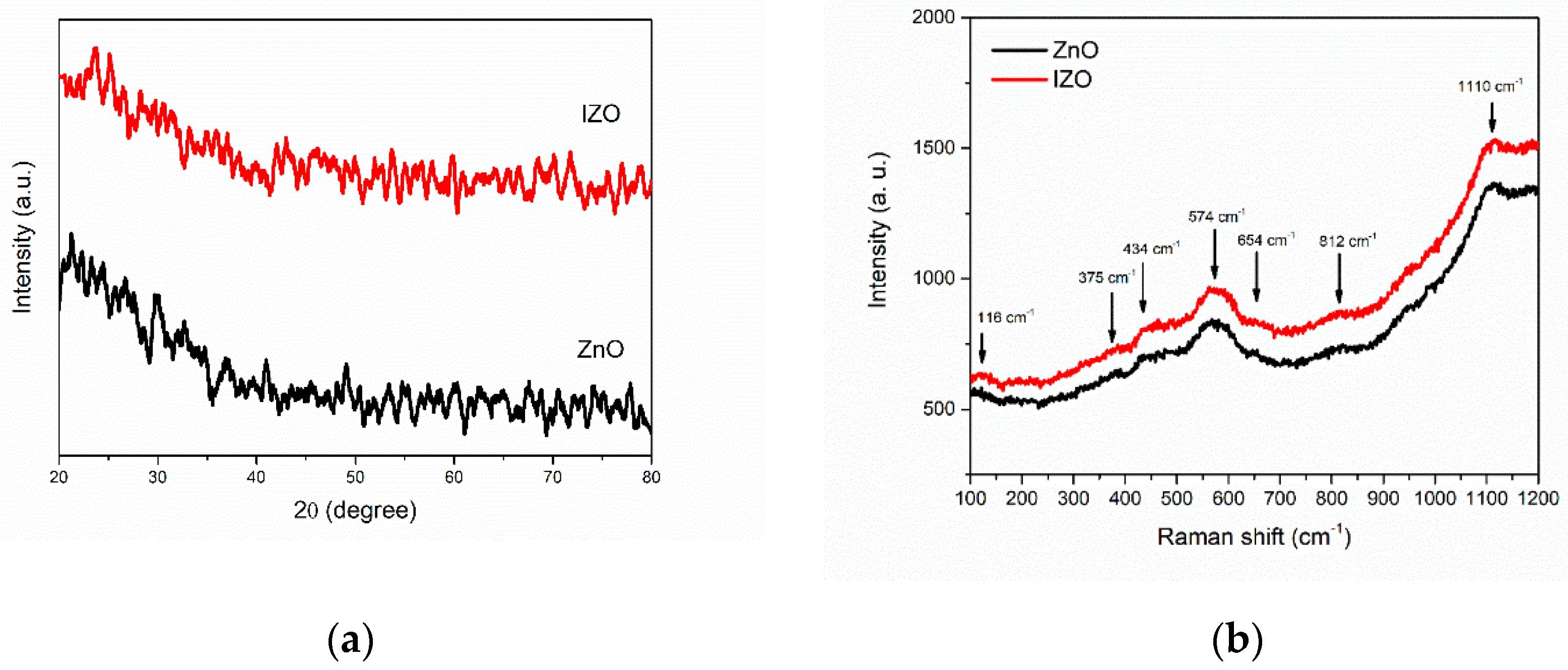
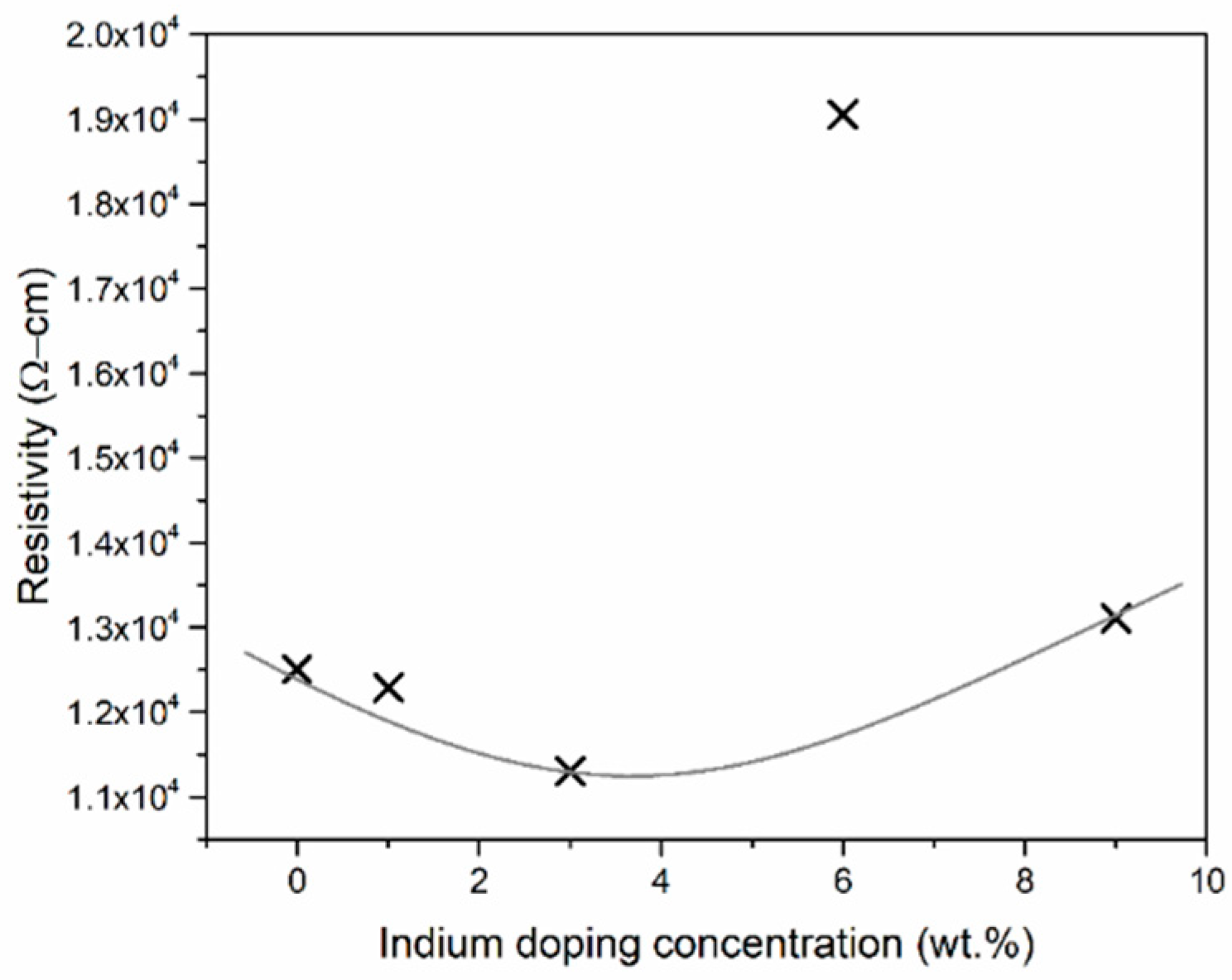
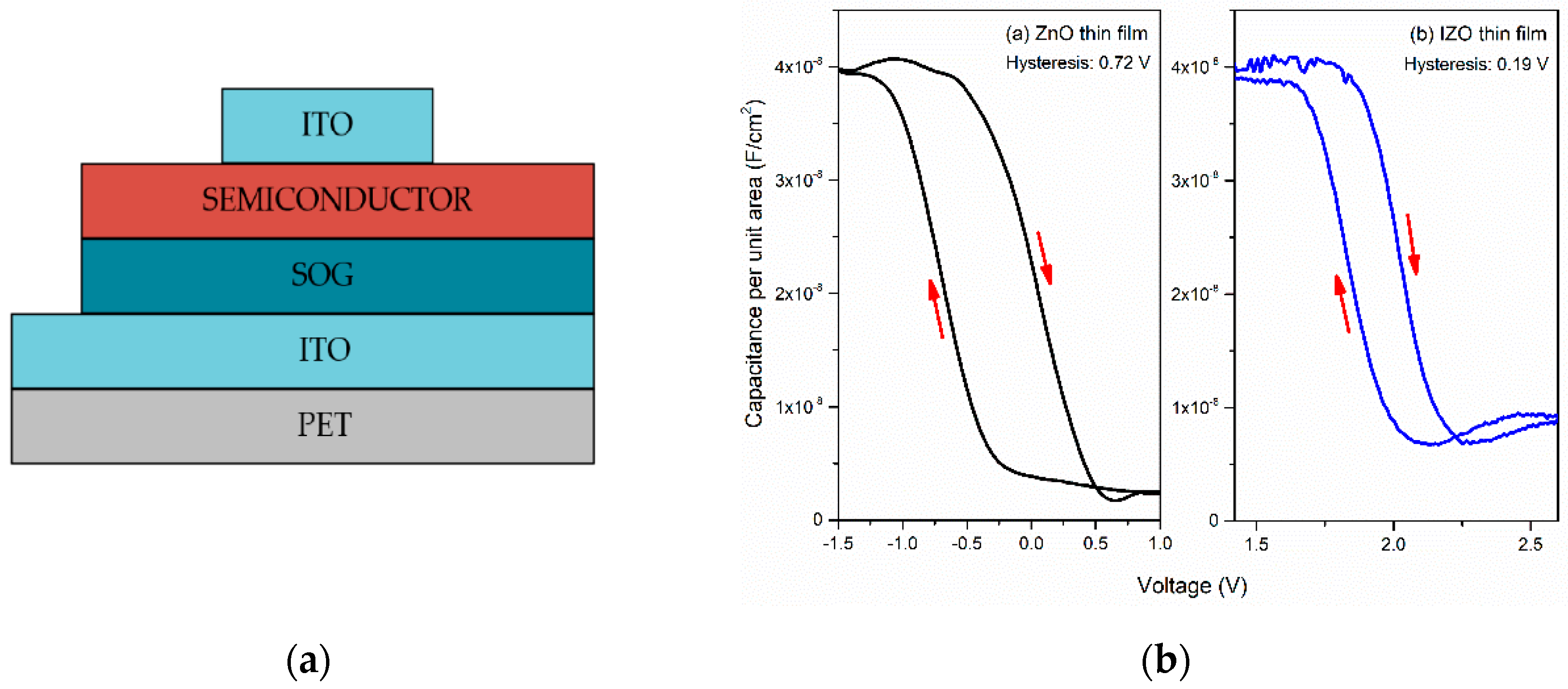
3. Results and Discussion
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jo, J.-W.; Kang, S.-H.; Heo, J.S.; Kim, Y.-H.; Park, S.K. Flexible metal oxide semiconductor devices made by solution methods. Chem. Eur. J. 2020, 26, 9126. [Google Scholar] [CrossRef] [PubMed]
- Jilani, A.; Abdel-wahab, M.S.; Hammad, A.H. Advance deposition techniques for thin film and coating, modern technologies for creating the thin-film systems and coatings. Mod. Technol. Creat. Thin-Film Syst. Coat. 2017, 2, 137–149. [Google Scholar] [CrossRef]
- Leng, J.; Wang, Z.; Wang, J.; Wu, H.-H.; Yan, G.; Li, X.; Guo, H.; Liu, X.; Zhang, Q.; Guo, Z. Advances in nanostructures fabricated via spray pyrolysis and their applications in energy storage and conversion. Chem. Soc. Rev. 2019, 48, 3015–3072. [Google Scholar] [PubMed]
- Bretos, I.; Jiménez, R.; Ricote, J.; Calzada, M.L. Low-temperature solution approaches for the potential integration of ferroelectric oxide films in flexible electronics. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2020, 67, 1967–1979. [Google Scholar] [CrossRef] [PubMed]
- Cheong, K.Y.; Mohammed, N.; Ramanan, S. Electrical and optical studies of ZnO:Ga thin films fabricated via the sol–gel technique. Thin Solid Films 2002, 410, 142–146. [Google Scholar] [CrossRef]
- Kuznetsov, D.A.; Han, B.; Yu, Y.; Rao, R.R.; Hwang, J.; Román-Leshkov, Y.; Shao-Horn, Y. Tuning redox transition via inductive effect in metal oxides and complexes, and implications in oxygen electrocatalysis. Joule 2018, 2, 225–244. [Google Scholar] [CrossRef]
- Etourneau, J.; Portier, J. The role of the inductive effect in solid state chemistry how the chemist can use it to modify both the structural and the physical properties of the materials. J. Alloys Compd. 1992, 188, 1–7. [Google Scholar] [CrossRef]
- Lee, S.Y. Comprehensive review on amorphous oxide semiconductor thin film transistor. Trans. Electr. Electron. Mater. 2020, 21, 235–248. [Google Scholar] [CrossRef]
- Lee, J.-H.; Oh, K.; Jung, K.; Wilson, K.C.; Lee, M.-J. Tuning the morphology and properties of nanostructured Cu-ZnO thin films using a two-step sputtering technique. Metals 2020, 10, 437. [Google Scholar] [CrossRef]
- Oliveira, A.G.; Andrade, J.d.L.; Montanha, M.C.; Lima, S.M.; da Cunha Andrade, L.H.; Winkler Hechenleitner, A.A.; Gómez Pineda, E.A.; Fernandes de Oliveira, D.M. Decontamination and disinfection of wastewater by photocatalysis under UV/visible light using nano-catalysts based on Ca-doped ZnO. J. Environ. Manag. 2019, 240, 485–493. [Google Scholar] [CrossRef]
- Santhosh Kumar, A.; Huang, N.M.; Nagaraja, H.S. Influence of Sn doping on photoluminescence and photoelectrochemical properties of ZnO nanorod arrays, Electron. Mater. Lett. 2014, 10, 753–758. [Google Scholar] [CrossRef]
- Medjaldi, M.; Touil, O.; Boudine, B.; Zaabat, M.; Halimi, O.; Sabais, M.; Ozyuzer, L. Study of undoped and indium doped ZnO thin films deposited by sol gel method. Silicon 2018, 10, 2577–2584. [Google Scholar] [CrossRef]
- Cuscó, R.; Alarcón-Lladó, E.; Ibáñez, J.; Artús, L.; Jiménez, J.; Wang, B.; Callahan, M.J. Temperature dependence of Raman scattering in ZnO. Phys. Rev. B. 2007, 75, 165202. [Google Scholar] [CrossRef]
- Dominguez, M.A.; Martinez, J.; Monfil-Leyva, K.; Soto, S.; Netzahualcoyolt, C.; Moreno, M. Incorporation of ZnO nanoparticles on solution processed zinc oxide thin-film transistors. Trans. Electr. Electron. Mater. 2018, 19, 412–416. [Google Scholar] [CrossRef]
- Adamopoulos, G.; Bashir, A.; Gillin, W.P.; Georgakopoulos, S.; Shkunov, M.; Baklar, M.A.; Stingelin, N.; Bradley, D.D.C.; Anthopoulos, T.D. Structural and electrical characterization of ZnO films grown by spray pyrolysis and their application in thin-film transistors. Adv. Funct. Mater. 2011, 21, 525–531. [Google Scholar] [CrossRef]
- Adamapoulos, G.; Bashir, A.; Wöbkenberg, P.W.; Bradley, D.D.C.; Anthopoulos, T.D. Electronic properties of ZnO field-effect transistors fabricated by spray pyrolysis in air ambient. Appl. Phys. Lett. 2009, 95, 133507. [Google Scholar] [CrossRef]
- Gonçalves, A.S.; Marques de Lima, S.A.; Davolos, M.R. Gallium-doped zinc oxide prepared by the Pechini method. Eclet. Quím. 2001, 27, 293–304. [Google Scholar] [CrossRef]
- Ahn, B.D.; Jeon, H.-J.; Sheng, J.; Park, J.; Park, J.-S. A review on the recent developments of solution processes for oxide thin films transistors. Semicond. Sci. Technol. 2015, 30, 064001. [Google Scholar] [CrossRef]
- Dominguez, M.A.; Orduña-Diaz, A. Fully solution-processed zinc oxide MIS capacitors by ultrasonic spray pyrolysis in air ambient. J. Appl. Res. Technol. 2017, 15, 278–282. [Google Scholar] [CrossRef]
- Saha, J.K.; Bukke, R.N.; Mude, N.N.; Jang, J. Significant improvement of spray pyrolyzed ZnO thin film by precursor optimization for high mobility thin film transistors. Sci. Rep. 2020, 10, 8999. [Google Scholar] [CrossRef]
- Todorov, T.; Hillhouse, H.W.; Aazou, S.; Sekkat, Z.; Vigil-Galán, O.; Deshmukh, S.D.; Agrawal, R.; Bourdais, S.; Valdés, M.; Arnou, P.; et al. Solution-based synthesis of kesterite thin film semiconductors. J. Phys. Energy. 2020, 2, 012003. [Google Scholar] [CrossRef]
- Hennek, J.W.; Smith, J.; Yan, A.; Kim, M.-G.; Zhao, W.; Dravid, V.P.; Facchetti, A.; Marks, T.J. Oxygen “getter” effects on microstructure and carrier transport in low temperature combustion-processed a-InXZnO (X = Ga, Sc, Y, La) transistors. J. Am. Chem. Soc. 2013, 135, 10729–10741. [Google Scholar] [CrossRef] [PubMed]
- Barret, R. Importance and Evaluation of the pKa. In Therapeutical Chemistry, 1st ed.; Barret, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 21–51. [Google Scholar]
- Zeumault, A.; Subramanian, V. Use of high-k encapsulation to improve mobility in trap-limited metal-oxide semiconductors. Phys. Status Solidi B 2017, 254, 1700124. [Google Scholar] [CrossRef]
- Song, Y.; Katsman, A.; Butcher, A.L.; Paine, D.C.; Zaslavsky, A. Temporal and voltage stress stability of high performance indium-zinc-oxide thin film transistors. Solid State Electron. 2017, 136, 43–50. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceron, S.; Orduña-Diaz, A.; Dominguez, M.A. Influence of Inductive Effect in Organic Residuals Content in IZO Thin Films and the Performance on the Behavior of MIS Capacitors on Plastic. Eng. Proc. 2021, 4, 9. https://doi.org/10.3390/Micromachines2021-09551
Ceron S, Orduña-Diaz A, Dominguez MA. Influence of Inductive Effect in Organic Residuals Content in IZO Thin Films and the Performance on the Behavior of MIS Capacitors on Plastic. Engineering Proceedings. 2021; 4(1):9. https://doi.org/10.3390/Micromachines2021-09551
Chicago/Turabian StyleCeron, Sonia, Abdu Orduña-Diaz, and Miguel A. Dominguez. 2021. "Influence of Inductive Effect in Organic Residuals Content in IZO Thin Films and the Performance on the Behavior of MIS Capacitors on Plastic" Engineering Proceedings 4, no. 1: 9. https://doi.org/10.3390/Micromachines2021-09551
APA StyleCeron, S., Orduña-Diaz, A., & Dominguez, M. A. (2021). Influence of Inductive Effect in Organic Residuals Content in IZO Thin Films and the Performance on the Behavior of MIS Capacitors on Plastic. Engineering Proceedings, 4(1), 9. https://doi.org/10.3390/Micromachines2021-09551





