Bridging Virtual and Reality: Testing Design of Experiment Procedures with Simulated and Experimental Data †
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Determination of Indium by ICP–MS
3.1.1. Optimisation of ICP Torch Position Employing Box–Behnken Design
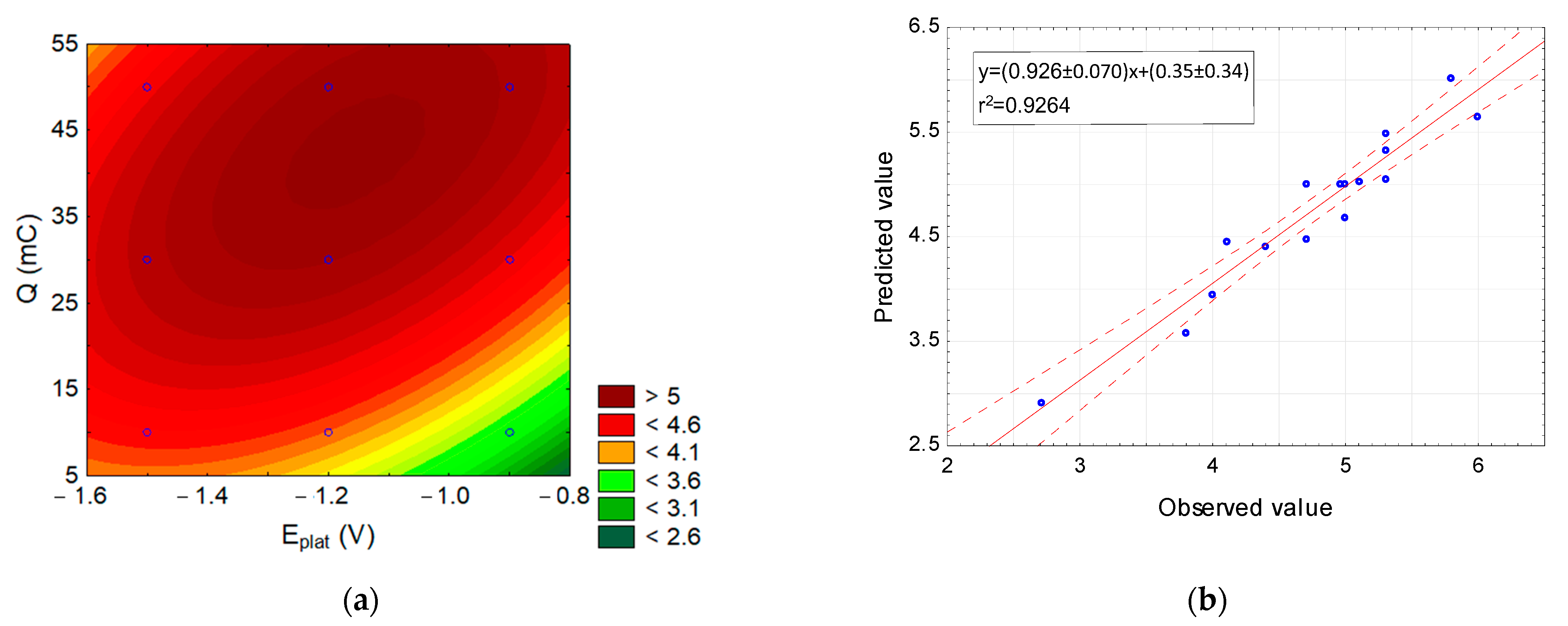
3.1.2. Optimisation of ICP Torch Position Employing Central Composite Design
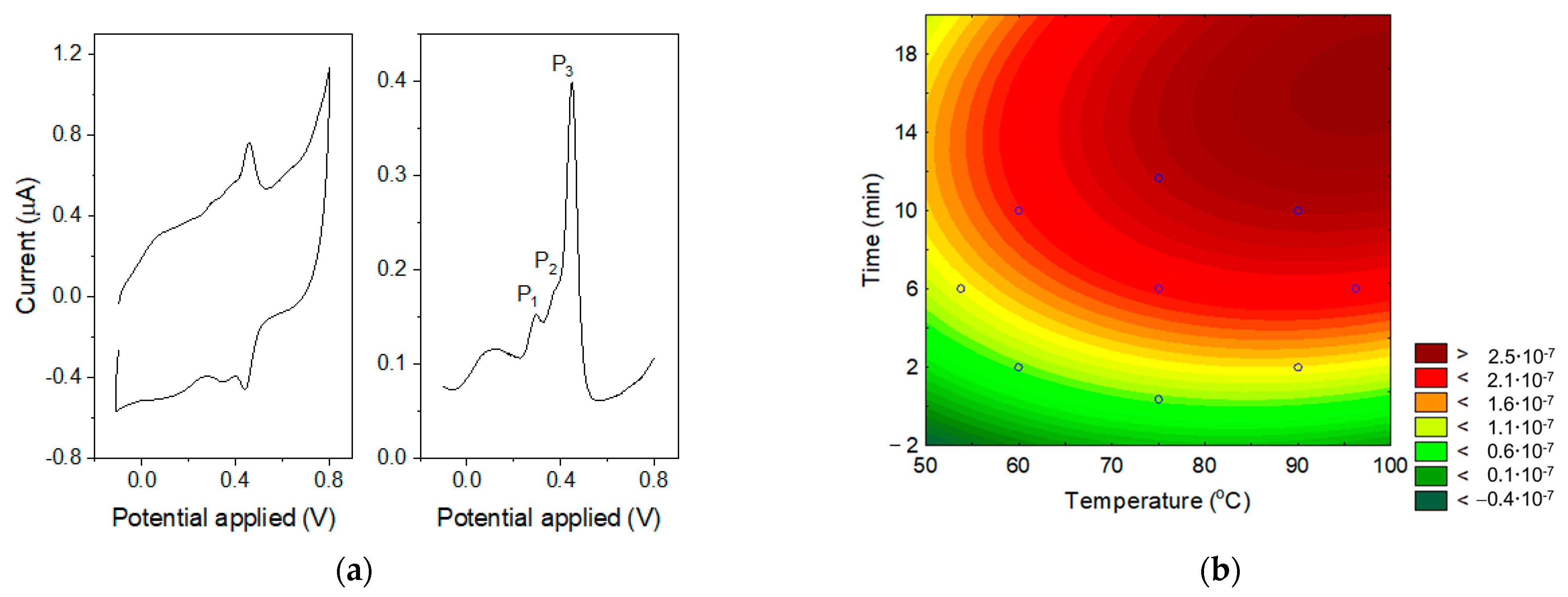
3.2. Determination of Germanium by Catalytic Adsorptive Striping Voltammetry
3.3. Determination of Antioxidant Properties Using Voltammetry
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Develve. Available online: https://develve.net/Central%20Composite%20design.html. (accessed on 12 August 2023).
- Bezerra, M.; Santelli, R.; Oliveira, E.; Villar, L.; Escaleira, L. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Managh, A.; Reid, P.; Knox, M. Development and Use of “ICP-MS TuneSim”: A Software App that Allows Students to Simulate Tuning an Inductively Coupled Plasma Mass Spectrometer. J. Chem. Educ. 2018, 95, 2059–2063. [Google Scholar] [CrossRef]
- Krolicka, A.; Zarebski, J.; Bobrowski, A. Catalytic Adsorptive Stripping Voltammetric Determination of Germanium Employing the Oxidizing Properties of V(IV)-HEDTA Complex and Bismuth-Modified Carbon-Based Electrodes. Membranes 2021, 11, 524. [Google Scholar] [CrossRef] [PubMed]
- Krolicka, A.; Szczurkowska, A.; Mochalski, P.; Malata, G. Preparation, Characterization, and Activation of Natural Glassy Carbon Paste Electrodes as New Sensors for Determining the Total Antioxidant Capacity of Plant Extracts. Membranes 2022, 12, 1193. [Google Scholar] [CrossRef] [PubMed]
- Chevion, S.; Chevion, M. Antioxidant status and human health—Use of cyclic voltammetry for the evaluation of the antioxidant capacity of plasma and of edible plants. Ann. New York Acad. Sci. 2000, 899, 308–325. [Google Scholar] [CrossRef] [PubMed]
- Chevion, S.; Roberts, M.; Chevion, M. The use of cyclic voltammetry for the evaluation of antioxidant capacity. Free. Radic. Biol. Med. 2000, 28, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Kulić, Ž.; Lehner, M.D.; Dietz, G.P.H. Ginkgo biloba leaf extract EGb 761® as a paragon of the product by process concept. Front. Pharmacol. 2022, 13, 1007746. [Google Scholar] [CrossRef] [PubMed]


| No. | CCD | CCD-CSP | BBD | ||||||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | x | y | z | x | y | z | |
| 1 | −1 | −1 | -1 | −1 | −1 | −1 | −1 | −1 | 0 |
| 2 | −1 | −1 | 1 | −1 | −1 | 1 | 1 | −1 | 0 |
| 3 | −1 | 1 | −1 | −1 | 1 | −1 | −1 | 1 | 0 |
| 4 | −1 | 1 | 1 | −1 | 1 | 1 | 1 | 1 | 0 |
| 5 | 1 | −1 | −1 | 1 | −1 | −1 | −1 | 0 | −1 |
| 6 | 1 | −1 | 1 | 1 | −1 | 1 | 1 | 0 | −1 |
| 7 | 1 | 1 | −1 | 1 | 1 | −1 | −1 | 0 | 1 |
| 8 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 |
| 9 | −1.68 | 0 | 0 | −1 | 0 | 0 | 0 | −1 | −1 |
| 10 | 1.68 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | −1 |
| 11 | 0 | −1.68 | 0 | 0 | −1 | 0 | 0 | −1 | 1 |
| 12 | 0 | 1.68 | 0 | 0 | 1 | 0 | 0 | 1 | 1 |
| 13 | 0 | 0 | −1.68 | 0 | 0 | −1 | 0 | 0 | 0 |
| 14 | 0 | 0 | 1.68 | 0 | 0 | 1 | 0 | 0 | 0 |
| 15 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 16 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 17 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 18 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 19 | 0 | 0 | 0 | 0 | 0 | 0 | - | - | - |
| Parameter | BBD1 | BBD2 | BBD3 | CCD–CSP | CCD | |
|---|---|---|---|---|---|---|
| Torch position | X (mm) | 3.2 | 3.2 | 3.2 | 3.3 | 3.3 |
| Y (mm) | 3.0 | 3.0 | 3.0 | 3.1 | 3.2 | |
| Z (mm) | 0.3 | 0.1 | 0.2 | -0.9 | -0.9 | |
| Intensity of 115In signal (cps) | predicted | 10,463 ± 346 | 10,496 ± 3472 | 10,458 ± 354 | 10,837 ± 1160 | 11,043 ± 1098 |
| measured | 10,417 ± 43 | 10,506 ± 65 | 10,432 ± 54 | 11,877 ± 65 | 11,879 ± 69 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Królicka, A.; Szczurkowska, A. Bridging Virtual and Reality: Testing Design of Experiment Procedures with Simulated and Experimental Data. Eng. Proc. 2023, 48, 25. https://doi.org/10.3390/CSAC2023-14888
Królicka A, Szczurkowska A. Bridging Virtual and Reality: Testing Design of Experiment Procedures with Simulated and Experimental Data. Engineering Proceedings. 2023; 48(1):25. https://doi.org/10.3390/CSAC2023-14888
Chicago/Turabian StyleKrólicka, Agnieszka, and Anna Szczurkowska. 2023. "Bridging Virtual and Reality: Testing Design of Experiment Procedures with Simulated and Experimental Data" Engineering Proceedings 48, no. 1: 25. https://doi.org/10.3390/CSAC2023-14888
APA StyleKrólicka, A., & Szczurkowska, A. (2023). Bridging Virtual and Reality: Testing Design of Experiment Procedures with Simulated and Experimental Data. Engineering Proceedings, 48(1), 25. https://doi.org/10.3390/CSAC2023-14888






