Development of a Portable Electrochemical Platform with Chip-Integrated Gold Electrodes for Detection of Pharmaceutical Pollutants †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Fabrication and Characterization of Chip-Integrated Gold Electrodes
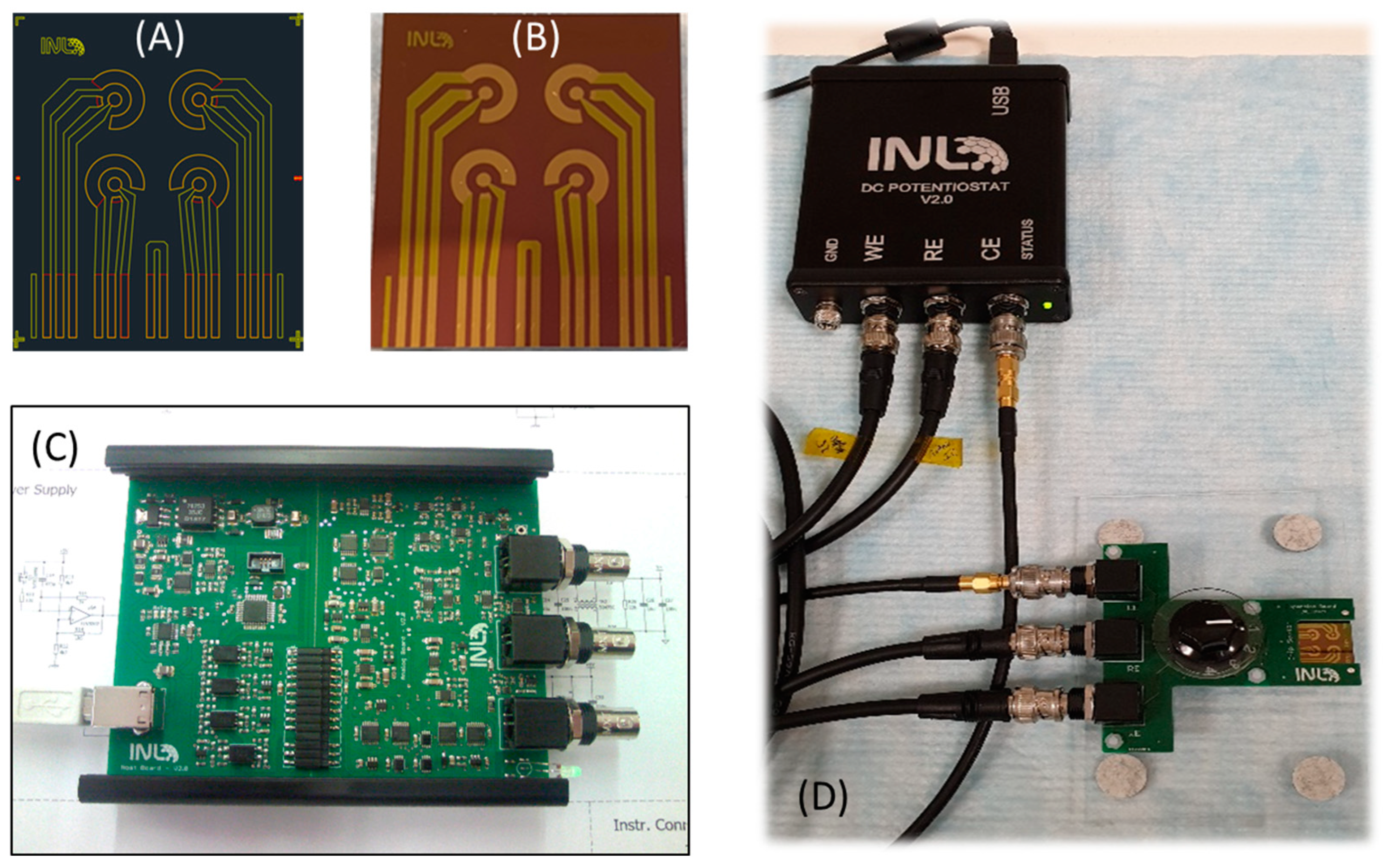
2.2.1. Design and Development of Electrochemical Cell-Chips
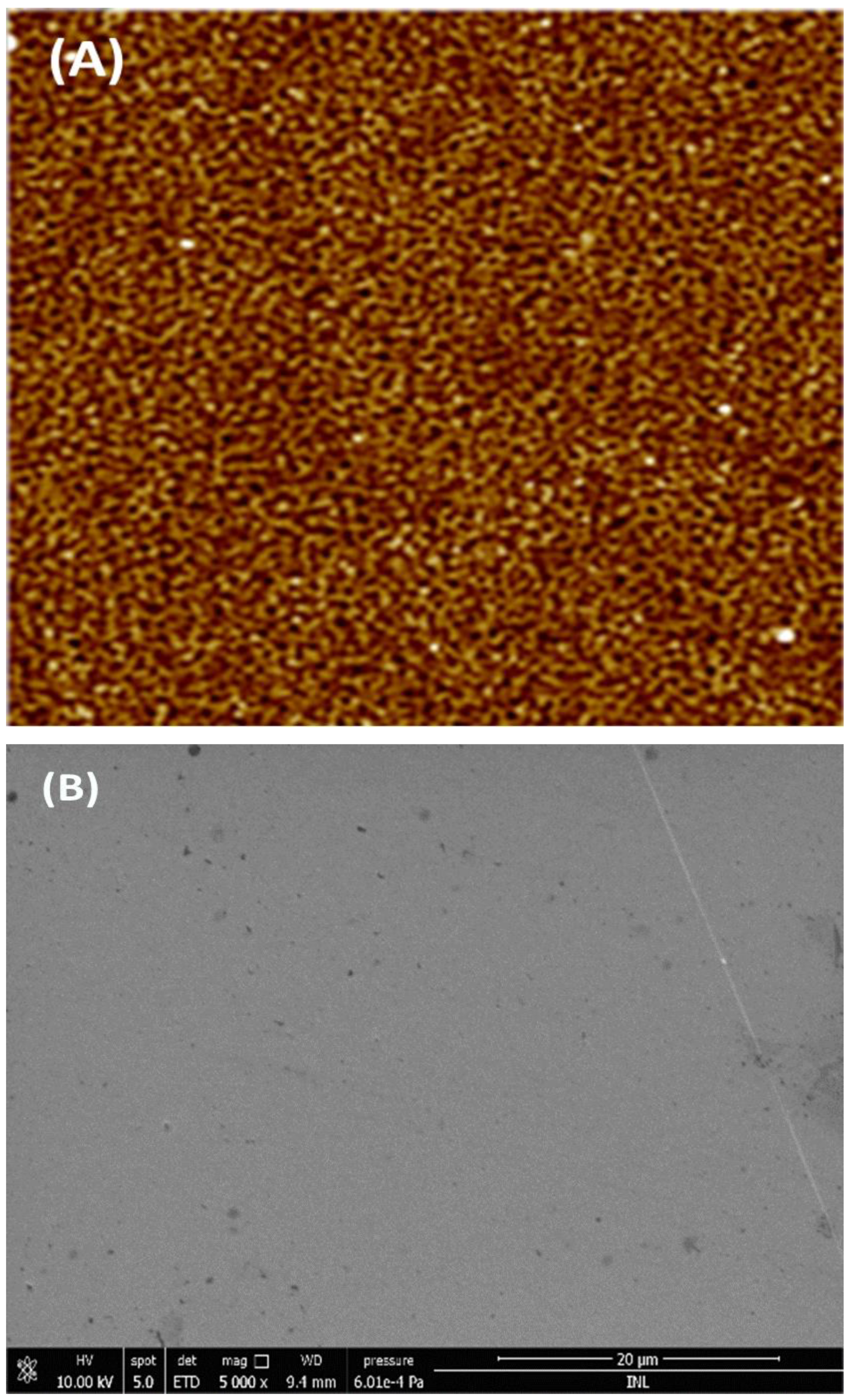
2.2.2. Characterization of the Surface Morphology
2.3. Electrochemical Characterization and Determination of Acetaminophen
3. Results and Discussion
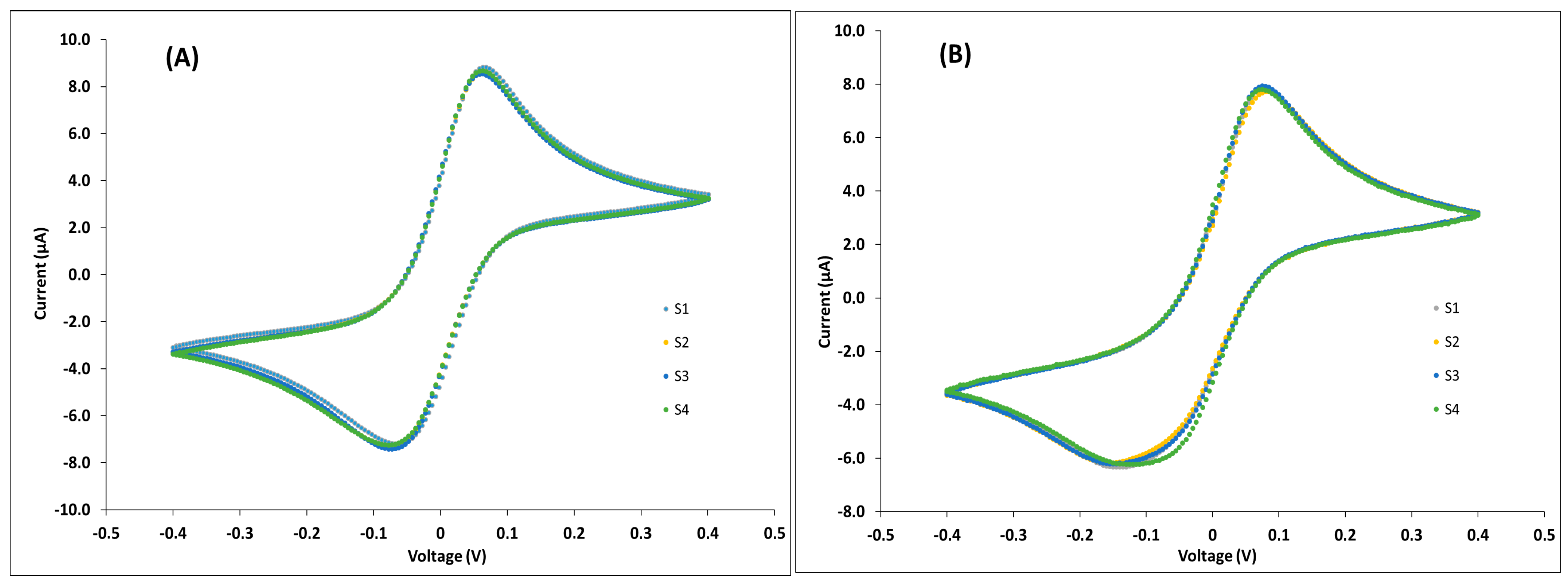
3.1. Characterization of the the ECCs Surface by AFM, SEM, and CV
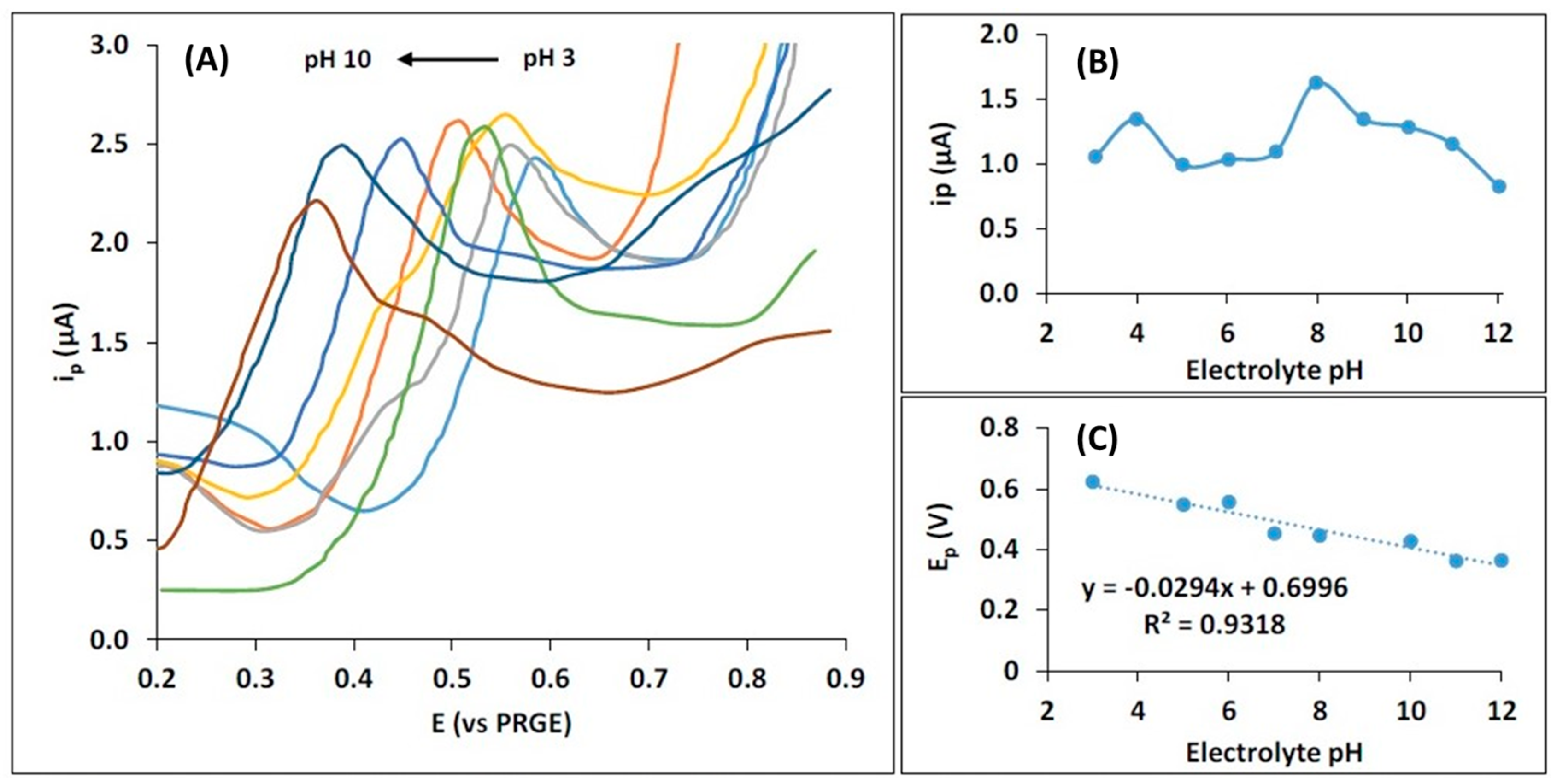
3.2. Optimization of Acetaminophen Electroanalysis by the ECCs with the EPP
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalambate, P.K.; Rao, Z.; Dhanjai; Wu, J.; Shen, Y.; Boddula, R.; Huang, Y. Electrochemical (bio)sensors go green. Biosens. Bioelectron. 2020, 163, 112270. [Google Scholar] [CrossRef] [PubMed]
- Torrinha, Á.; Morais, S. Electrochemical (bio)sensors based on carbon cloth and carbon paper: An overview. TrAC Trends Anal. Chem. 2021, 142, 116324. [Google Scholar] [CrossRef]
- Verlicchi, P.; Al Aukidy, M.; Zambello, E. Occurrence of pharmaceutical compounds in urban wastewater: Removal, mass load and environmental risk after a secondary treatment—A review. Sci. Total Environ. 2012, 429, 123–155. [Google Scholar] [CrossRef]
- Koagouw, W.; Arifin, Z.; Olivier, G.W.J.; Ciocan, C. High concentrations of paracetamol in effluent dominated waters of Jakarta Bay, Indonesia. Mar. Pollut. Bull. 2021, 169, 112558. [Google Scholar] [CrossRef]
- Ebele, A.J.; Abou-Elwafa Abdallah, M.; Harrad, S. Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerg. Contam. 2017, 3, 1–16. [Google Scholar] [CrossRef]
- Gholivand, M.B.; Amiri, M. Simultaneous detection of dopamine and acetaminophen by modified gold electrode with polypyrrole/aszophloxine film. J. Electroanal. Chem. 2012, 676, 53–59. [Google Scholar] [CrossRef]
- Jia, L.; Zhang, X.H.; Li, Q.; Wang, S.F. Determination of acetaminophen by square wave voltammetry at a gold electrode modified by 4-amino-2-mercaptopyrimidine self-assembled monolayers. J. Anal. Chem. 2007, 62, 266–269. [Google Scholar] [CrossRef]
- Menon, S.; Jesny, S.; Girish Kumar, K. A voltammetric sensor for acetaminophen based on electropolymerized-molecularly imprinted poly(o-aminophenol) modified gold electrode. Talanta 2018, 179, 668–675. [Google Scholar] [CrossRef]
- Menon, S.; Kumar, K.G. Simultaneous Voltammetric Determination of Acetaminophen and Its Fatal Counterpart Nimesulide by Gold Nano/L-Cysteine Modified Gold Electrode. J. Electrochem. Soc. 2017, 164, B482. [Google Scholar] [CrossRef]
- dos Santos, P.L.; Katic, V.; Loureiro, H.C.; dos Santos, M.F.; dos Santos, D.P.; Formiga, A.L.B.; Bonacin, J.A. Enhanced performance of 3D printed graphene electrodes after electrochemical pre-treatment: Role of exposed graphene sheets. Sens. Actuators B Chem. 2019, 281, 837–848. [Google Scholar] [CrossRef]
- Akhter, S.; Basirun, W.J.; Alias, Y.; Johan, M.R.; Bagheri, S.; Shalauddin, M.; Ladan, M.; Anuar, N.S. Enhanced amperometric detection of paracetamol by immobilized cobalt ion on functionalized MWCNTs—Chitosan thin film. Anal. Biochem. 2018, 551, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Afkhami, A.; Khoshsafar, H.; Bagheri, H.; Madrakian, T. Preparation of NiFe2O4/graphene nanocomposite and its application as a modifier for the fabrication of an electrochemical sensor for the simultaneous determination of tramadol and acetaminophen. Anal. Chim. Acta 2014, 831, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Tadayon, F.; Vahed, S.; Bagheri, H. Au-Pd/reduced graphene oxide composite as a new sensing layer for electrochemical determination of ascorbic acid, acetaminophen and tyrosine. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 68, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Mulyasuryani, A.; Tjahjanto, R.T.; Andawiyah, R.a. Simultaneous Voltammetric Detection of Acetaminophen and Caffeine Base on Cassava Starch—Fe3O4 Nanoparticles Modified Glassy Carbon Electrode. Chemosensors 2019, 7, 49. [Google Scholar] [CrossRef]
- Kang, X.; Wang, J.; Wu, H.; Liu, J.; Aksay, I.A.; Lin, Y. A graphene-based electrochemical sensor for sensitive detection of paracetamol. Talanta 2010, 81, 754–759. [Google Scholar] [CrossRef]
- Miller, N.J.; Miller, C.J. Statistics and Chemometrics for Analytical Chemistry, 6th ed.; Pearson Education Limited: Harlow, UK, 2010. [Google Scholar]
- Serrano, N.; Castilla, Ò.; Ariño, C.; Diaz-Cruz, M.S.; Díaz-Cruz, J.M. Commercial Screen-Printed Electrodes Based on Carbon Nanomaterials for a Fast and Cost-Effective Voltammetric Determination of Paracetamol, Ibuprofen and Caffeine in Water Samples. Sensors 2019, 19, 4039. [Google Scholar] [CrossRef]
- Deroco, P.B.; Fatibello-Filho, O.; Arduini, F.; Moscone, D. Effect of Different Carbon Blacks on the Simultaneous Electroanalysis of Drugs as Water Contaminants Based on Screen-printed Sensors. Electroanalysis 2019, 31, 2145–2154. [Google Scholar] [CrossRef]
- Saciloto, T.R.; Cervini, P.; Cavalheiro, E.T.G. Simultaneous voltammetric determination of acetaminophen and caffeine at a graphite and polyurethane screen-printed composite electrode. J. Braz. Chem. Soc. 2013, 24, 1461–1468. [Google Scholar] [CrossRef]
- Camargo, J.R.; Andreotti, I.A.A.; Kalinke, C.; Henrique, J.M.; Bonacin, J.A.; Janegitz, B.C. Waterproof paper as a new substrate to construct a disposable sensor for the electrochemical determination of paracetamol and melatonin. Talanta 2020, 208, 120458. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, J.H.; Tran, V.-K.; Ko, E.; Park, C.H.; Chung, W.S.; Seong, G.H. Determination of acetaminophen using functional paper-based electrochemical devices. Sens. Actuators B Chem. 2016, 232, 514–522. [Google Scholar] [CrossRef]
- Fanjul-Bolado, P.; Lamas-Ardisana, P.J.; Hernández-Santos, D.; Costa-García, A. Electrochemical study and flow injection analysis of paracetamol in pharmaceutical formulations based on screen-printed electrodes and carbon nanotubes. Anal. Chim. Acta 2009, 638, 133–138. [Google Scholar] [CrossRef] [PubMed]


| Sample | Spiking Level (mM) | Recovery (%) | RSD (%) |
|---|---|---|---|
| Wastewater | 0.6 | 96.6 | 4.1 |
| River water | 0.6 | 93.0 | 3.6 |
| 0.3 | 93.6 | 9.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavares, M.; Torrinha, Á.; Queirós, R.; Piteira, J.; Delerue-Matos, C.; Morais, S. Development of a Portable Electrochemical Platform with Chip-Integrated Gold Electrodes for Detection of Pharmaceutical Pollutants. Eng. Proc. 2023, 48, 50. https://doi.org/10.3390/CSAC2023-14900
Tavares M, Torrinha Á, Queirós R, Piteira J, Delerue-Matos C, Morais S. Development of a Portable Electrochemical Platform with Chip-Integrated Gold Electrodes for Detection of Pharmaceutical Pollutants. Engineering Proceedings. 2023; 48(1):50. https://doi.org/10.3390/CSAC2023-14900
Chicago/Turabian StyleTavares, Miguel, Álvaro Torrinha, Raquel Queirós, João Piteira, Cristina Delerue-Matos, and Simone Morais. 2023. "Development of a Portable Electrochemical Platform with Chip-Integrated Gold Electrodes for Detection of Pharmaceutical Pollutants" Engineering Proceedings 48, no. 1: 50. https://doi.org/10.3390/CSAC2023-14900
APA StyleTavares, M., Torrinha, Á., Queirós, R., Piteira, J., Delerue-Matos, C., & Morais, S. (2023). Development of a Portable Electrochemical Platform with Chip-Integrated Gold Electrodes for Detection of Pharmaceutical Pollutants. Engineering Proceedings, 48(1), 50. https://doi.org/10.3390/CSAC2023-14900








