Abstract
This paper presents the synthesis of fluorescein-modified hydrogel materials. The obtained materials were characterized using FT-IR spectroscopy. Then, their sorption capacity in distilled water and Ringer’s liquid was determined. Using digital microscopy, the morphology of the obtained systems was characterized.
1. Introduction
Hydrogels, owing to their distinctive physical and chemical properties, have found extensive applications across diverse industrial domains. The hydrogel properties of materials depend on the degree and nature of cross-linking and the degree of crystallinity of the polymer [1,2]. Notably, hydrogels exhibit a pronounced potential for biomedical applications, such as tissue engineering and drug delivery, which is attributable to their hydrophilicity, superabsorbent capacity, biodegradability, and biocompatibility. Despite their hydrophilic nature, these materials are non-soluble in aqueous media. Of significance, hydrogels display non-toxicity, an absence of tissue damage, and an absence of inflammatory responses or thrombogenicity [3]. Hydrogel materials are commonly composed of natural polymers like collagen, gelatin, or cellulose, forming three-dimensional networks of hydrophilic polymer chains capable of absorbing substantial fluids, water, or bodily fluids. The structure and properties of hydrogel materials can be tailored through the selection of appropriate biomaterials, cross-linking methodologies, and fabrication techniques [4,5,6].
Hydrogels, constituted by intelligent polymers, exhibit a sensitivity to alterations in their external environment. These smart hydrogels contain functional groups that are formed as a result of non-covalent interactions, such as hydrogen bonds, hydrophobic interactions, or electrostatic interactions. The most common stimuli for hydrogel responsiveness encompass light, temperature, electromagnetic fields, ultrasound, and chemical factors, e.g., pH, as well as biological triggers like antigens or DNA [7,8]. Physical and chemical stimuli are harnessed to elicit various structural reactions from intelligent polymers. One or more of these stimuli can induce modifications in diverse properties, including phase transitions, shape alterations, optical, mechanical, and molecular attributes, surface energy, reaction rates, and permeability of the polymer system. A notable characteristic of temperature-sensitive polymers involves the presence of hydrophobic groups such as propyl, ethyl, and methyl groups [9,10]. When temperature-sensitive polymers reach the lower critical solution temperature (LCST), a phase transition is observed within the polymer structure. pH-responsive polymers represent another pivotal group of intelligent polymers. These polymers consist of polymer chains with ionic groups that can donate or accept protons as a result of changes in the pH of the environment. When these materials are exposed to these factors, properties such as the porosity and hydrophilicity of hydrogels can regulate the loading and release of drugs in a controlled manner, thereby finding utility in drug delivery systems [11,12]. In this work, fluorescein-modified hydrogel materials were obtained, which can be used as various types of sensors in many industries. An example of unmodified and dye-containing materials is presented in Figure 1.

Figure 1.
Fluorescein-modified hydrogels (a) and unmodified hydrogels (b).
2. Materials and Methods
2.1. Materials
The primary reagents employed in this study encompassed poly(vinyl alcohol) (PVA, in crystalline powder form, hydrolyzed to a degree of 87–89%, with a molecular weight range of 13,000–23,000), poly(ethylene glycol) (PEG, in powder form, possessing an average molecular weight of 6000), diacrylate poly(ethylene glycol) (PEGDA, a cross-linking agent, with an average molecular weight of Mn = 700 g/mol), and 2-hydroxy-2-methylpropiophenone (a photoinitiator with a purity of 97% and a density d = 1.077 g/mL). Additionally, fluorescein (in the form of the free acid) was procured from Sigma Aldrich (Saint Louis, MO, USA). All the acquired reagents were of analytical grade purity.
2.2. Synthesis of Hydrogel Materials
The hydrogel materials were obtained through the photopolymerization process. First, specified amounts of PVP and PVA were mixed. Fluorescein was then added with various amounts of cross-linking agent. After thorough mixing, a photoinitiator was introduced into the reaction mixture, which was then placed in a mold for polymerization. The entire process was carried out at ambient temperature using a 180 W EMITA VP-60 UV lamp with a wavelength of λ = 320 nm. The duration of the polymerization process for each material was set to 5 min. After synthesis, the materials were subjected to complex drying until they reached a solid state. The synthesized materials were then subjected to a physicochemical characterization including infrared spectroscopy and sorption analysis. In addition, their surface morphology was evaluated. The composition of the hydrogels is presented in Table 1.

Table 1.
Composition of the hydrogel materials obtained.
2.3. FT-IR Infrared Spectroscopy Analysis
The investigation involving Fourier Transform Infrared (FT-IR) spectroscopy was executed utilizing the Thermo Scientific Nicolet iS5 spectrophotometer equipped with an ATR attachment. The spectra were recorded in the range 3600–500 cm−1 (32 scans, resolution 4.0 cm−1). The measurement was carried out at room temperature.
2.4. Analysis of Sorption Capacity
The sorption capacity of the polymeric materials was evaluated through the determination of their swelling coefficient. For each distinct material specimen, a circular sample measuring 1 cm in diameter was meticulously prepared and its initial mass was ascertained. Subsequently, these samples were introduced into separate volumes of distilled water and Ringer solution (each maintained in 50 mL of the corresponding liquid). Post a designated incubation period of 24 h and 48 h, the samples were, once again, weighed, thereby facilitating the computation of their swelling coefficient using the subsequent formula (Equation (1)).
α = (mt – m0)/m0
, where the components are as follows:
- α—swelling ratio, in g/g;
- mt—mass of swollen sample after time “t”, in g;
- m0—mass of dry sample (before the study), in g.
2.5. Microscopic Observations and Roughness Profile
Then, the surface morphology of the obtained materials was determined using an advanced VKX-7000 Keyence digital microscope. The observations were conducted for all the received materials at room temperature.
3. Results and Discussion
3.1. FT-IR Infrared Spectroscopy Analysis
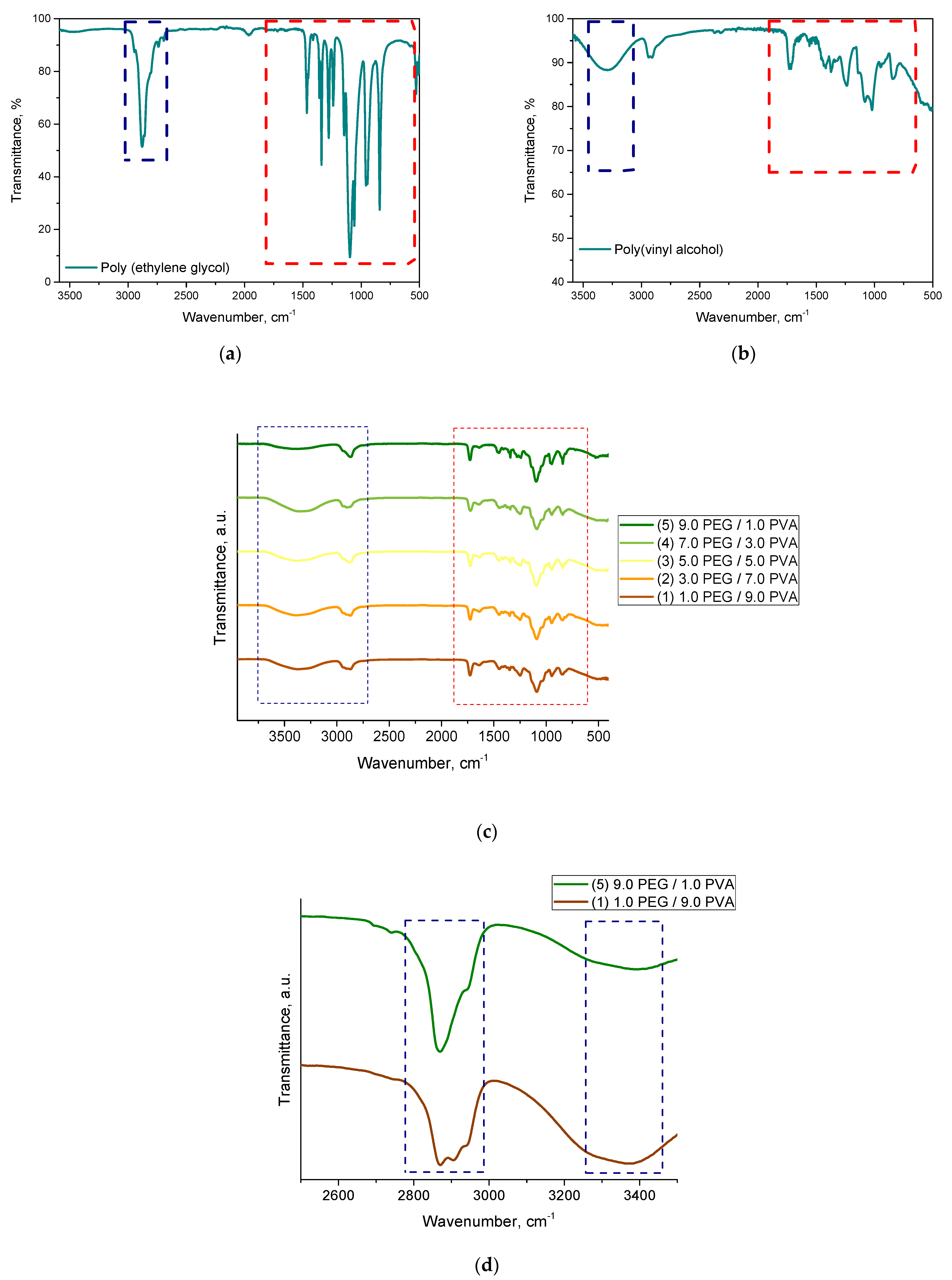
A spectroscopic analysis was performed to determine the chemical structure of the resulting hydrogels. The spectroscopic spectra are presented in Figure 2. All the hydrogel materials were analyzed. In addition, pure polymeric components such as PVA and PEG were tested as reference samples.
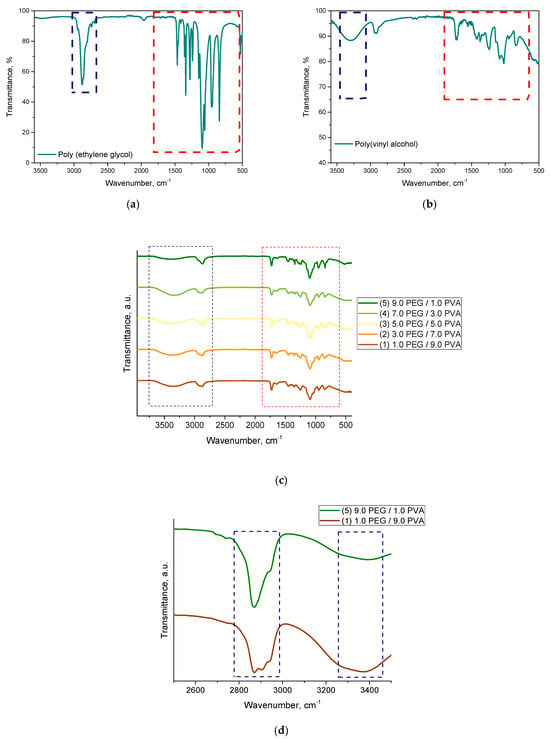
Figure 2.
The FTIR spectra of the hydrogel materials and the reference samples, as follows: PEG (a); PVA (b); hydrogels samples (c); and comparison of the two samples (d).
Based on the analysis, the occurrence of absorption bands characteristic of the polymeric components included in the developed hydrogel systems was confirmed. No significant differences were noted. All the spectroscopic spectra were similar to each other. They differed slightly in the intensity of the selected bands depending on the base solution used (the ratio of PEG to PVA). It was difficult to identify significant differences in the wavenumber range around 2000 cm−1 to 600 cm−1, because most of the absorption bands overlapped in this range for both polymers. This is indicated with the red box in Figure 2. However, we could also indicate the difference between the starting materials, as presented in Figure 2a,b. In the case of polyethylene glycol, we could distinguish an absorption band with a maximum around 2870 cm−1 corresponding to the C-H stretching vibrations of the -CH2 group. In the case of polyvinyl alcohol, on the other hand, a characteristic vibration of the -OH group was noted, with a maximum of about 3300 cm−1. These bands were compared for the samples containing extreme values of both of these components (sample 1 and 5). This is shown in Figure 2d. Accordingly, for sample 1, containing a higher proportion of PVA, we observed a high intensity of the band at 3300 cm−1, while, for sample 5, the intensity of this band decreased. In contrast, the band characteristic of polyethylene glycol stood out.
3.2. Analysis of Sorption Capacity
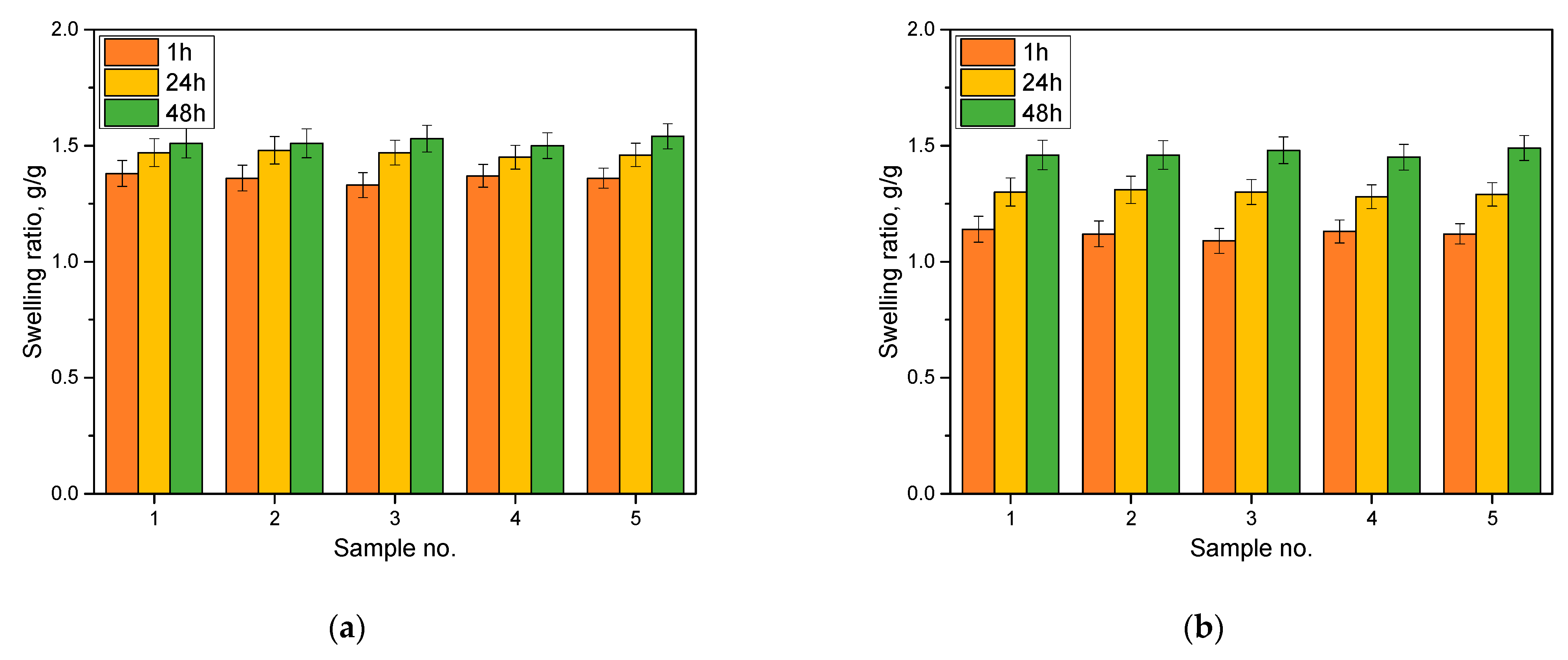
The results of the sorption capacity analysis are presented in Figure 3.
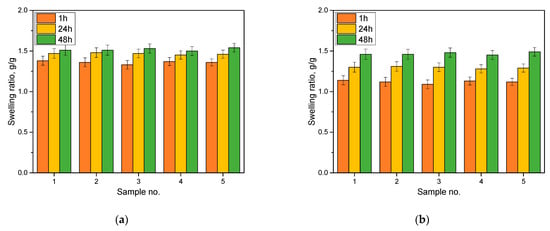
Figure 3.
Results of the sorption capacity analysis in distilled water (a) and Ringer solution (b). Number of repetitions, n = 3.
An analysis of the sorption capacity was carried out to determine whether the obtained polymeric materials were capable of absorbing water or aqueous solutions. Depending on their application, hydrogel materials should be characterized by an appropriate degree of swelling. For example, in biomedical applications, when we care about absorbing wound exudate, with materials intended as innovative dressings, sorptive capacity is extremely important. Other properties, on the other hand, will be required of materials intended for pharmaceutical or cosmetic applications. For engineered materials containing dye as a marker, the properties mentioned are equally important. During the swelling of hydrogel material, the polymer network is loosened, and the encapsulated substances can penetrate the polymer network, respectively, acting as a tracer. For the materials obtained, all of them showed sorption capacities of about 1.5 g/g in distilled water and 1.3 in Ringer’s liquid. The highest weight gain was observed within the first hour of analysis. In contact with the liquid, the hydrogel material swelled, and, with the passage of time, there was a filling of all the free spaces between the polymer chains, and the swelling coefficients changed only slightly. The swelling kinetics indicate that the first hour of contact between the material and the aqueous solution is the most significant. Then, with the passage of time, some stabilization occurs, and the hydrogel material accepts only insignificant batches of solution. For all the analyzed materials, smaller values of swelling coefficients were recorded for Ringer’s fluid than for the distilled water. It is likely that the ions present in this fluid may cause the occurrence of additional interactions in the polymer network, limiting the penetration of larger amounts of fluid, which is not the case with distilled water. These ions may create additional interactions between the polymer chains. When this happens, the polymer network increases its cross-linked density, and the penetrating liquid that penetrates the system has limited free spaces between the chains. This results in lower swelling coefficients. However, despite this phenomenon, the hydrogel materials placed in Ringer’s liquid also showed satisfactory values of swelling coefficients, testifying to their sorption capacity.
3.3. Microscopic Observations
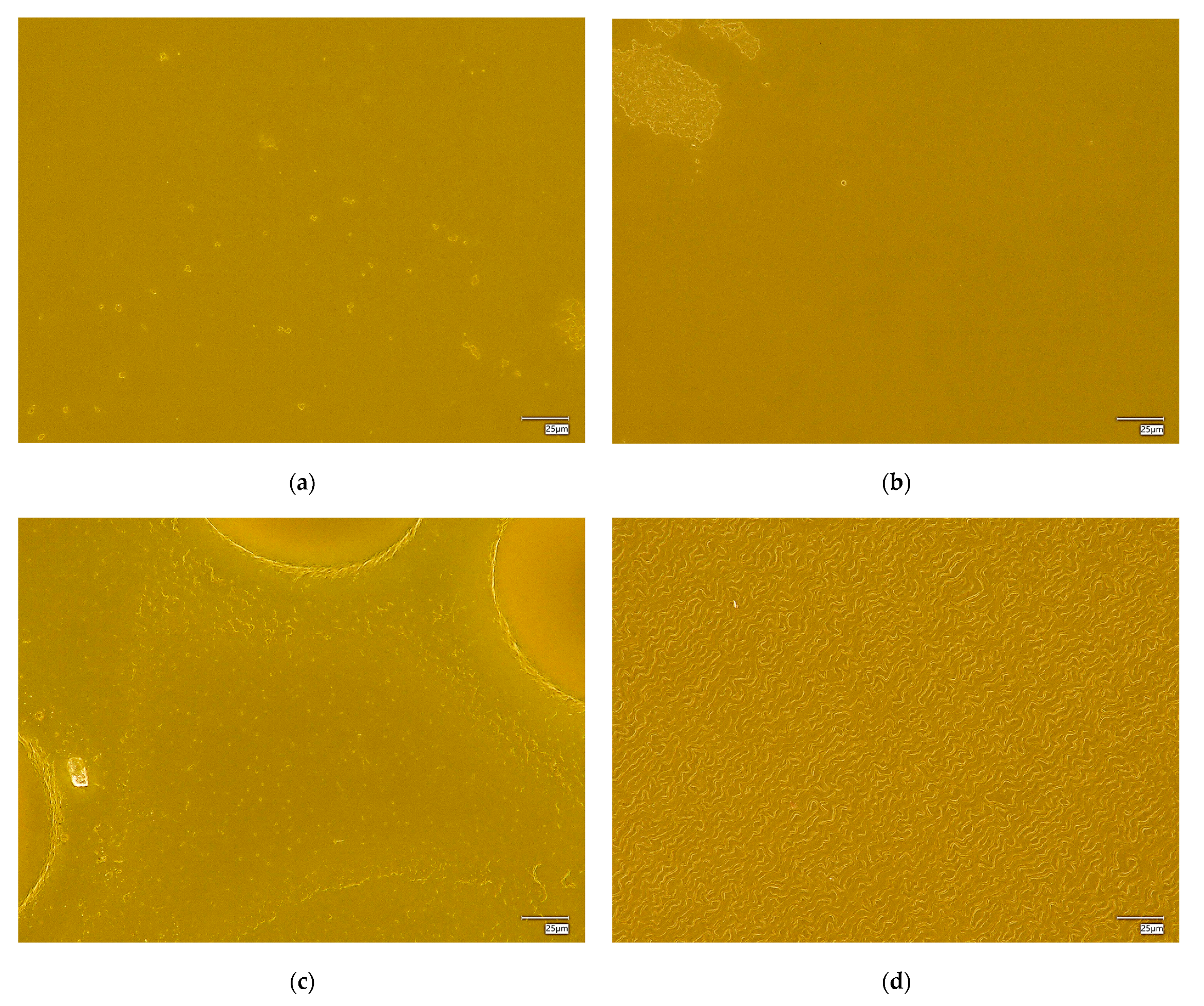
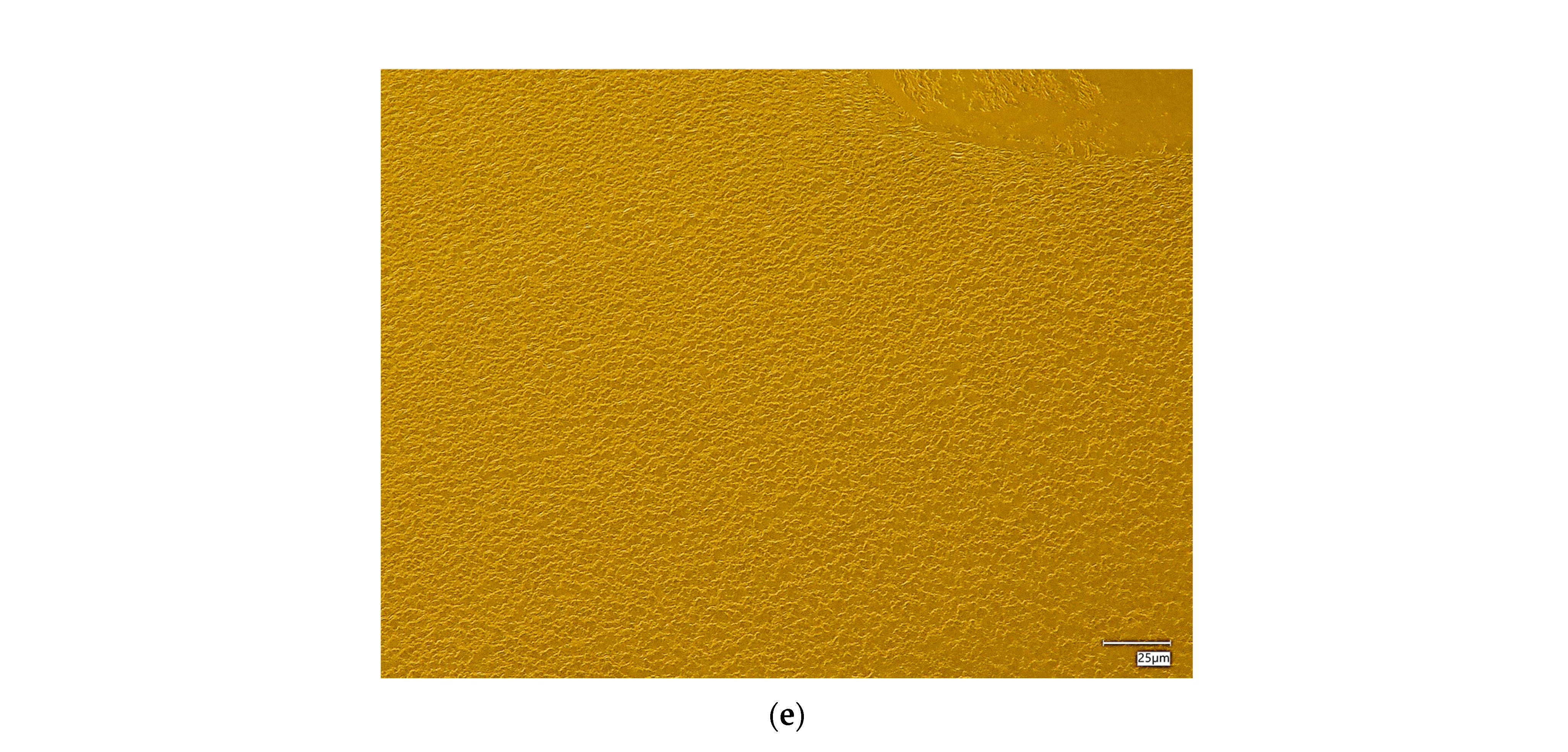
The surface morphology of the hydrogel materials is presented in Figure 4.
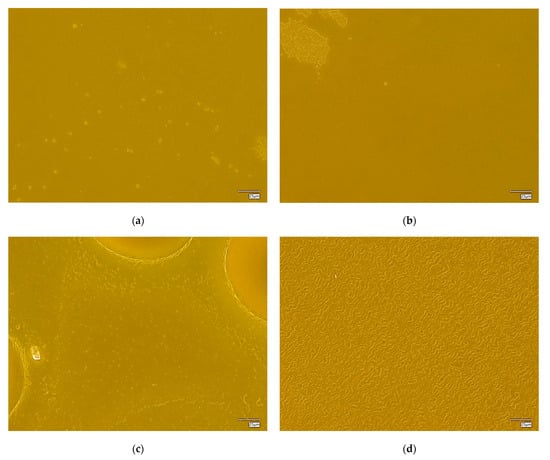
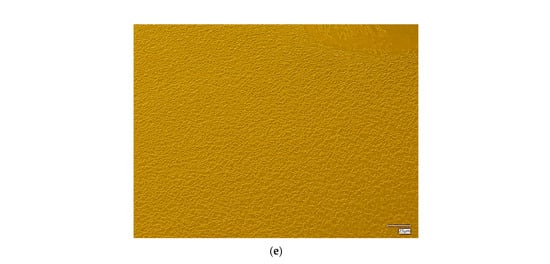
Figure 4.
Digital microscope images and roughness profile of the hydrogel materials, as follows: 1.0 PEG/9.0 PVA (a); 3.0 PEG/7.0 PVA (b); 5.0 PEG/5.0 PVA (c); 7.0 PEG/3.0 PVA (d); and 9.0 PEG/1.0 PVA (e).
The obtained materials are characterized by a rough surface structure, which is associated with the use of a selected amount of cross-linking agent. During polymerization, a polymer network of a specific packing density is formed, depending on the composition of the polymer matrix, mainly the cross-linking agent and the photoinitiator. In the case of all the materials held, a homogeneous surface without discontinuities was found, which shows the correct polymerization of these systems.
Some differences were also noted between the materials containing different amounts of selected polymers. It was found that, with an increasing ratio of PEG to PVA, the roughness and corrugation of the surface increased. Poly(vinyl alcohol) is a polymer with excellent film-forming properties, so, in the case of the samples containing a higher amount of it, a smoothing of the surface of the hydrogel material was observed, while, decreasing its amount in favor of PEG, caused the smoothing to disappear, making the material rougher. This is valuable information from an application point of view for these materials. Depending on the specific application, more or less surface roughness may be desired, which can be controlled and modified accordingly by changing the ratio of PEG to PVA, the base polymers.
4. Summary
- The selected photopolymerization technique enabled the production of hydrogel materials that were modified with a fluorescent dye.
- The obtained hydrogels were characterized according to their sorption capacities.
- The spectroscopic characterization showed no significant deviations between the materials studied.
- All the materials were characterized by a continuous, homogeneous polymerization surface structure, with an average degree of roughness.
Author Contributions
Conceptualization, M.B., B.T. and M.K.; methodology, M.B., B.T. and K.S.; software, M.B. and B.T.; validation, M.B. and B.T.; formal analysis, M.B., K.S., K.R., M.I., J.P., A.W. and D.W.; investigation, M.B., K.S., K.R., M.I., J.P., A.W. and D.W.; resources B.T.; data curation, B.T.; writing—original draft preparation, B.T., M.B., K.S. and K.G.; visualization, K.S.; supervision, B.T. and M.K.; project administration, B.T.; and funding acquisition, B.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was carried out within the SMART-MAT Functional Materials Science Club of the Faculty of Materials Engineering and Physics of Cracow University of Technology and as part of a project entitled “SmartGels”, financed by the FutureLab organization operating at CUT.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable for this paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhou, H.; Zhu, H.; Yang, X.; Zhang, Y.; Zhang, X.; Cui, K.; Shao, L.; Yao, J. Temperature/PH Sensitive Cellulose-Based Hydrogel: Synthesis, Characterization, Loading, and Release of Model Drugs for Potential Oral Drug Delivery. BioResources 2014, 10, 760–771. [Google Scholar] [CrossRef]
- Coșman, B.-P.; Bucătariu, S.-M.; Constantin, M.; Fundueanu, G. Temperature/PH-Sensitive Double Cross-Linked Hydrogels as Platform for Controlled Delivery of Metoclopramide. Gels 2022, 8, 824. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Yahya, R.; Hassan, A.; Yar, M.; Azzahari, A.D.; Selvanathan, V.; Sonsudin, F.; Abouloula, C.N. PH Sensitive Hydrogels in Drug Delivery: Brief History, Properties, Swelling, and Release Mechanism, Material Selection and Applications. Polymers 2017, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Mu, X.; Xu, Y.; Li, S.; Liu, X.; Lei, Z. Popcorn-Based Dual-Monomer Copolymerized Temperature/PH-Sensitive Core-Shell Hydrogels. J. Environ. Chem. Eng. 2023, 11, 109510. [Google Scholar] [CrossRef]
- Gao, C.; Ren, J.; Zhao, C.; Kong, W.; Dai, Q.; Chen, Q.; Liu, C.; Sun, R. Xylan-Based Temperature/PH Sensitive Hydrogels for Drug Controlled Release. Carbohydr. Polym. 2016, 151, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Markovic, M.D.; Panic, V.V.; Savic, S.I.; Ugrinovic, V.D.; Pjanovic, R.V.; Spasojevic, M.M.; Spasojevic, P.M. Biobased Thermo/PH Sensitive Poly(N-Isopropylacrylamide-Co-Crotonic Acid) Hydrogels for Targeted Drug Delivery. Microporous Mesoporous Mater. 2022, 335, 111817. [Google Scholar] [CrossRef]
- Kwon, S.S.; Kong, B.J.; Park, S.N. Physicochemical Properties of PH-Sensitive Hydrogels Based on Hydroxyethyl Cellulose–Hyaluronic Acid and for Applications as Transdermal Delivery Systems for Skin Lesions. Eur. J. Pharm. Biopharm. 2015, 92, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Sun, J.; Bing, L.; Cui, X.; Jia, B.; Bai, S. Fractal Features of Dual Temperature/PH-Sensitive Poly(N-Isopropylacrylamide-Co-Acrylic Acid) Hydrogels and Resultant Effects on the Controlled Drug Delivery Performances. Eur. Polym. J. 2022, 171, 111203. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Romero-Fierro, D.; Arcentales-Vera, B.; Palomino, K.; Magaña, H.; Bucio, E. Hydrogels Classification According to the Physical or Chemical Interactions and as Stimuli-Sensitive Materials. Gels 2021, 7, 182. [Google Scholar] [CrossRef] [PubMed]
- Geyik, G.; Işıklan, N. Synthesis, Characterization and Swelling Performance of a Temperature/PH-Sensitive κ-Carrageenan Graft Copolymer. Int. J. Biol. Macromol. 2020, 152, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar Physicochemical Properties: Pyrolysis Temperature and Feedstock Kind Effects. Rev. Environ. Sci. Bio/Technol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Johnson, B.D.; Beebe, D.J.; Crone, W.C. Effects of Swelling on the Mechanical Properties of a PH-Sensitive Hydrogel for Use in Microfluidic Devices. Mater. Sci. Eng. C 2004, 24, 575–581. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).