Development of Electric Power Generator by Using Hydrogen †
Abstract
:1. Introduction
2. Hydrogen Engine Generator Performance Test
2.1. Combustion Characteristics of Hydrogen
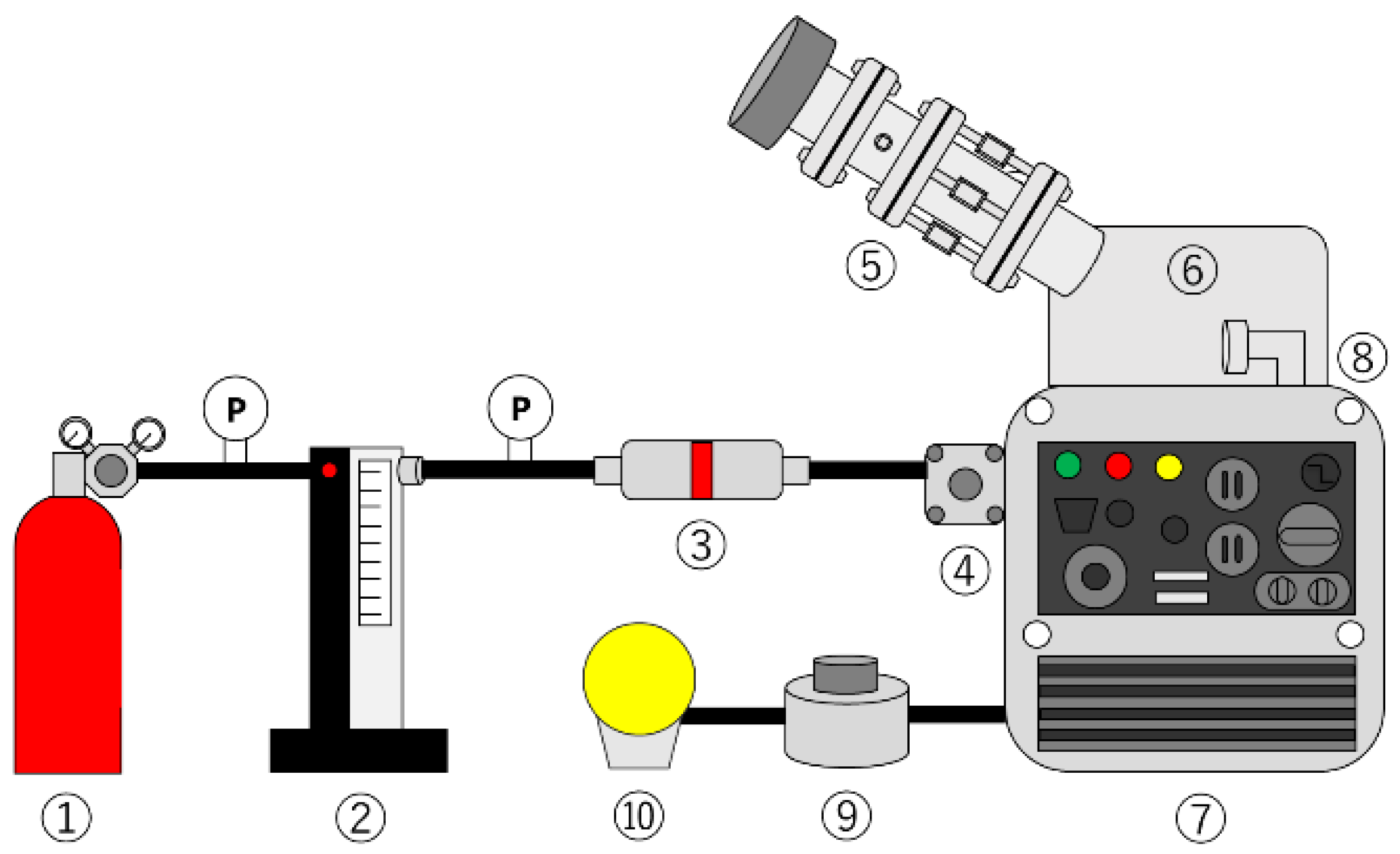
2.2. Experimental Method and Conditions
3. Hydrogen Generation
3.1. Principle of Hydrogen Generation
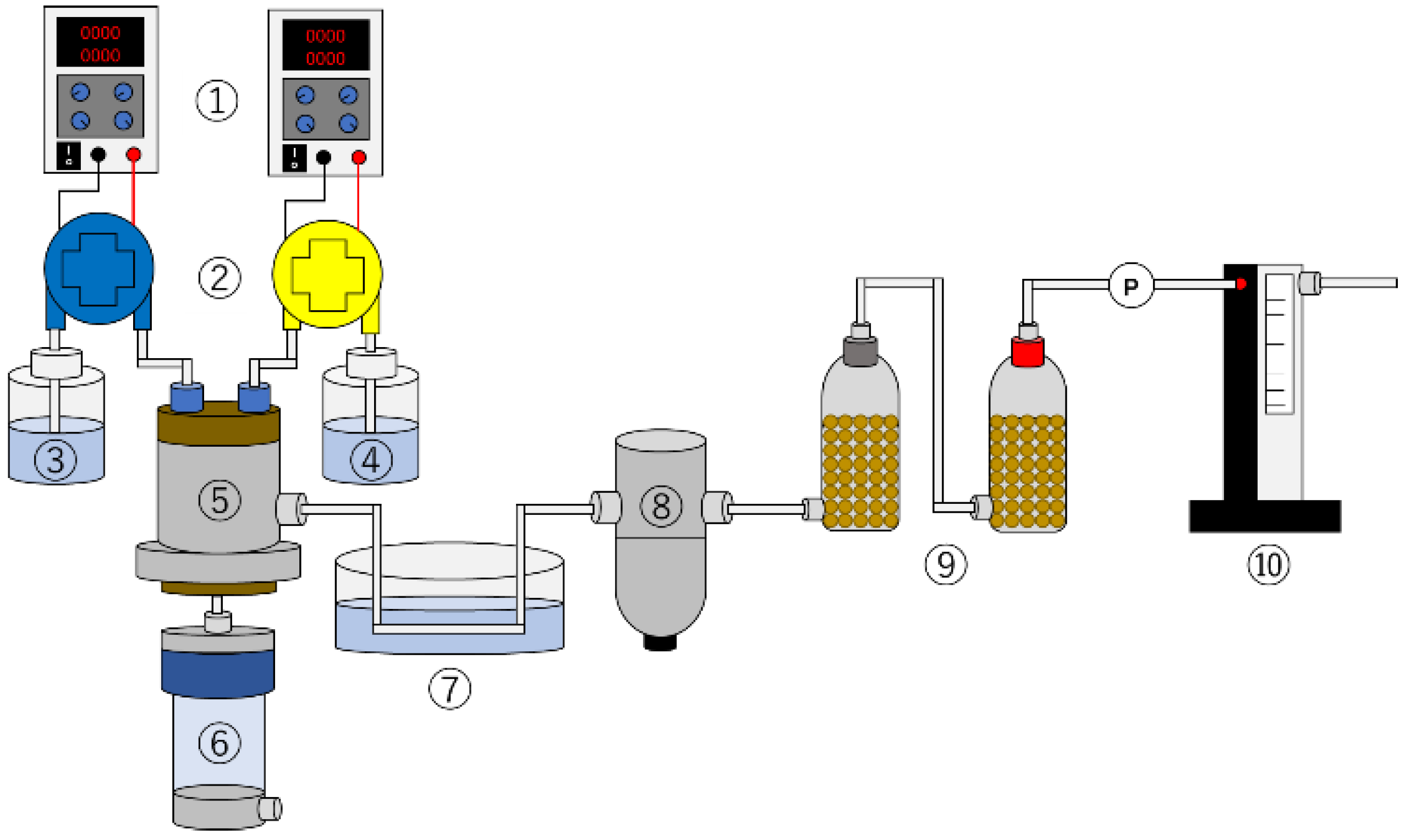
3.2. Experimental Method and Conditions
4. Results and Discussion
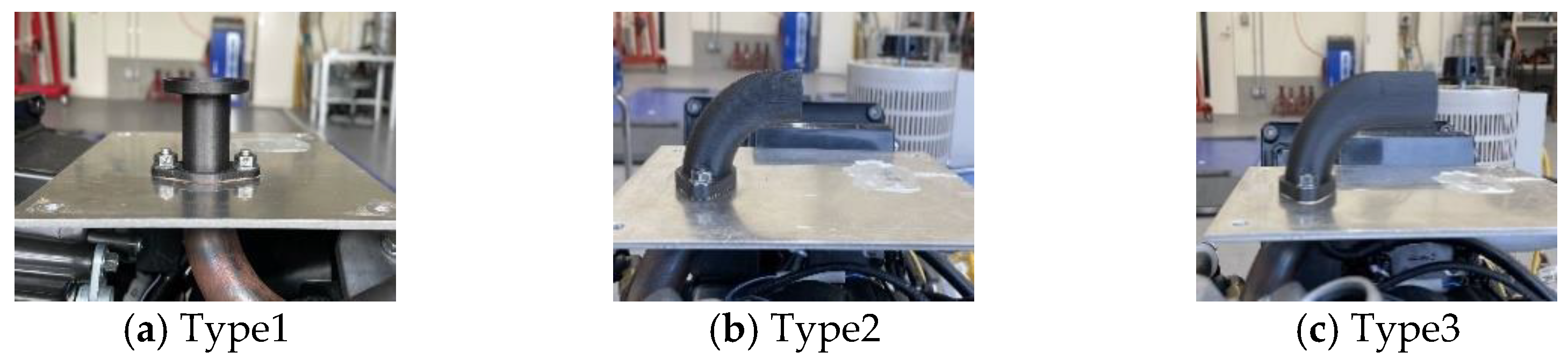
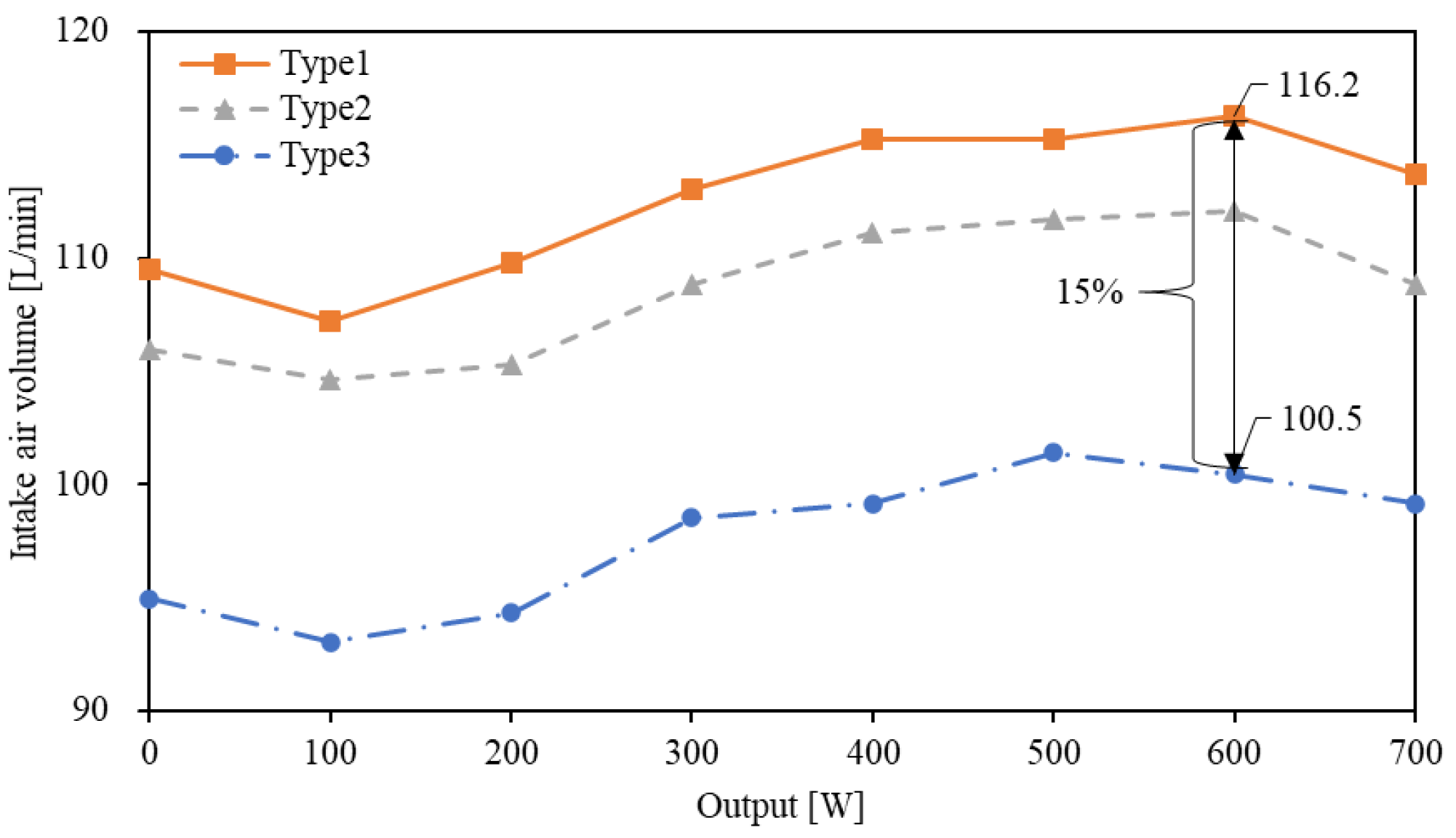
4.1. Influence of Intake Manifold Shape on Intake Air Volume
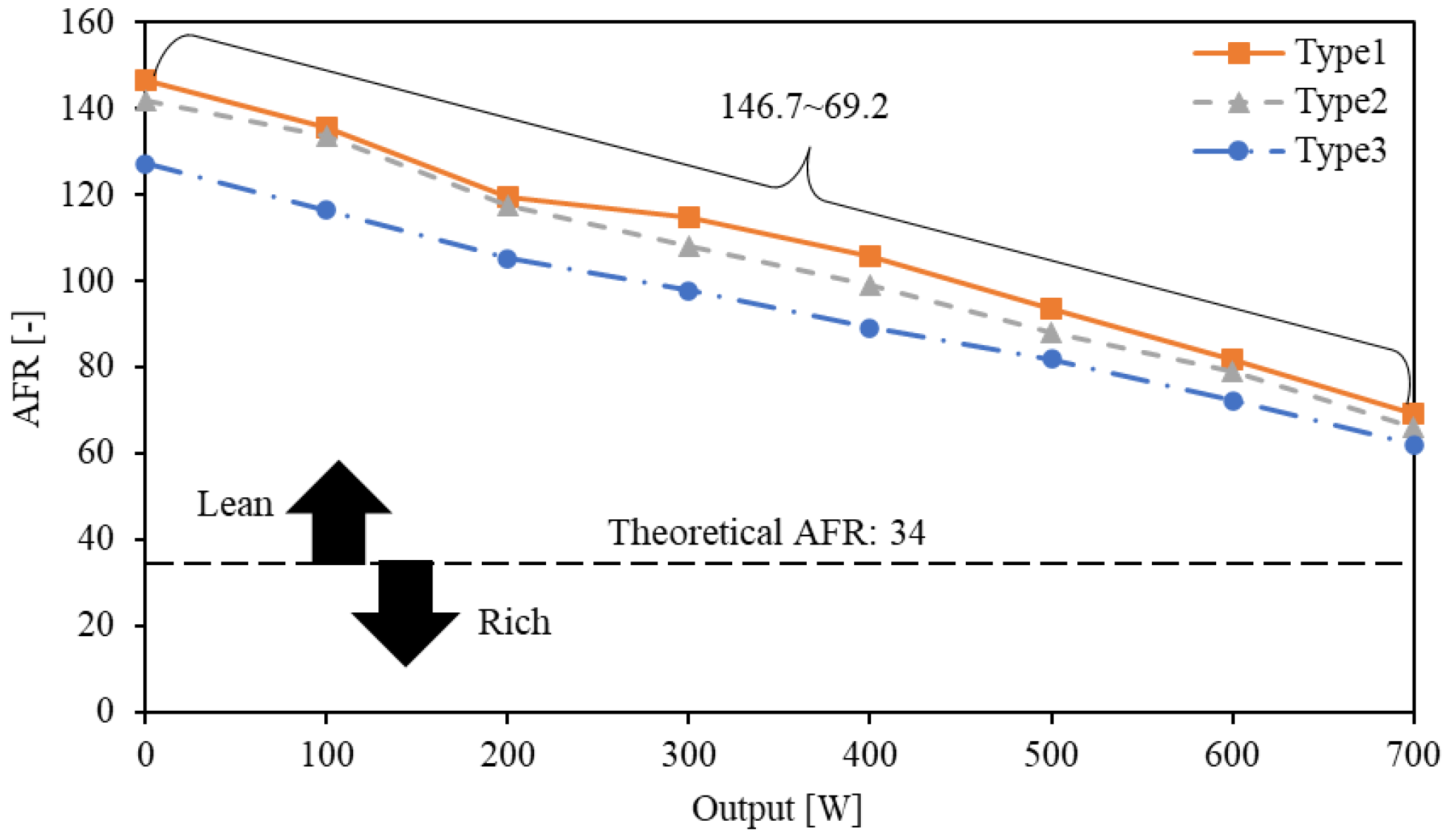
4.2. Influence of Intake Manifold Shape on AFR
4.3. Influence of Intake Manifold Shape on Thermal Efficiency
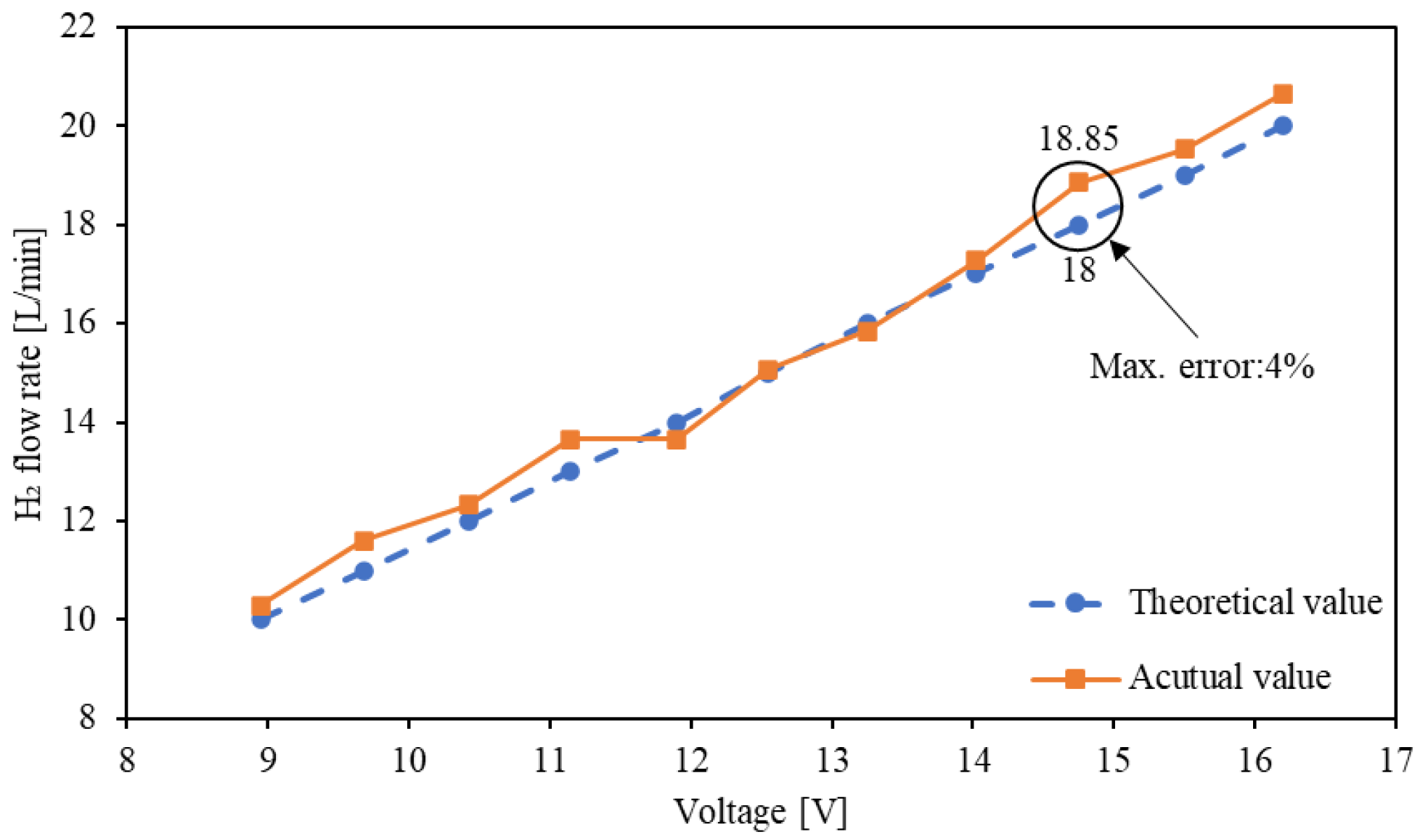
4.4. Measurement of Hydrogen Generation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sixth Assessment Report. Available online: https://www.ipcc.ch/report/ar6/syr/(04302023) (accessed on 1 April 2023).
- Yamane, K. Technology and Future of Reciprocating Hydrogen Internal Combustion Engine. Hydrog. Energy Syst. 2006, 31, 15–19. [Google Scholar]
- Fang, J.S. Development of Small Hydrogen Engine Generator. Master’s Thesis, Shizuoka Institute of Science and Technology, Fukuroi, Japan, 15 February 2022. [Google Scholar]
- Sato, Y. Safety Engineering. J. Inst. Electr. Install. Eng. Jpn. 2005, 44, 1–30. [Google Scholar]
- How to Make Hydrogen. Available online: https://www.enecho.meti.go.jp/about/special/johoteikyo/suiso_tukurikata.html(05052023) (accessed on 1 April 2023).
- Hoshi, N. Verification of Sodium Borohydride as Fuel for Fuel Cell Vehicles. Trans. Inst. Electr. Eng. Jpn. D 2011, 132, 30–40. [Google Scholar]
- Ishizuka, K. Research on Small Reactor for Emergency Hydrogen Generator Using Sodium Borohydride and Citric Acid. Graduation Thesis of Master Course, Tokyo University of Science, Tokyo, Japan, 15 February 2022. [Google Scholar]


| CH4 | H2 | |
| Molecular weight (g/mol) | 16 | 2 |
| Density (kg/m3) | 0.651 | 0.084 |
| Diffusion coefficient (m2/s) | 2.1 × 10−5 | 6.7 × 10−5 |
| Thermal conductivity (W/m·K) | 0.03 | 0.17 |
| Minimum ignition energy (mJ) | 0.28 | 0.02 |
| Flammable range (Vol.%) | 5~15 | 4~75 |
| Flame propagation speed (m/s) | 0.4 | 2.7 |
| Dimension | Type1 | Type2 | Type3 |
|---|---|---|---|
| Inner diameter (mm) | 21 | 23 | 23 |
| Outer diameter (mm) | 23 | 25 | 25 |
| Length (upper part) (mm) | 33 | 66 | 82 |
| Length (lower part) (mm) | 95 | 95 | 95 |
| Chemical Formula | NaBH4 | C6H8O7 |
|---|---|---|
| Shape | White solid crystal | White solid crystal |
| Molecular weight | 37.83 | 192.12 |
| Density (g/cm3) | 1.074 | 1.665 |
| Melting point (deg.) | 400 | 153 |
| Boiling point (deg.) | 500 | 175 |
| Solubility (g) | 55/H2O 100 (25 °C) | 73/H2O 100 (20 °C) |
| H2 Flow | Voltage (NaBH4) | NaBH4 | Voltage (C6H8O7) | C6H8O7 |
|---|---|---|---|---|
| L/min | V | L/min | V | L/min |
| 10 | 8.05 | 0.0116 | 8.95 | 0.0172 |
| 11 | 8.65 | 0.0128 | 9.68 | 0.0189 |
| 12 | 9.25 | 0.0139 | 10.42 | 0.0206 |
| 13 | 9.90 | 0.0151 | 11.15 | 0.0224 |
| 14 | 10.50 | 0.0162 | 11.90 | 0.0241 |
| 15 | 11.10 | 0.0174 | 12.55 | 0.0258 |
| 16 | 11.70 | 0.0186 | 13.25 | 0.0275 |
| 17 | 12.30 | 0.0197 | 14.02 | 0.0292 |
| 18 | 12.85 | 0.0209 | 14.75 | 0.0310 |
| 19 | 13.60 | 0.0220 | 15.50 | 0.0327 |
| 20 | 13.99 | 0.0232 | 16.20 | 0.0344 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, N.; Takeuchi, Y.; Amano, K.; Fukuda, K. Development of Electric Power Generator by Using Hydrogen. Eng. Proc. 2023, 55, 22. https://doi.org/10.3390/engproc2023055022
Zhu N, Takeuchi Y, Amano K, Fukuda K. Development of Electric Power Generator by Using Hydrogen. Engineering Proceedings. 2023; 55(1):22. https://doi.org/10.3390/engproc2023055022
Chicago/Turabian StyleZhu, Ning, Yuta Takeuchi, Katsuhiro Amano, and Kazuhito Fukuda. 2023. "Development of Electric Power Generator by Using Hydrogen" Engineering Proceedings 55, no. 1: 22. https://doi.org/10.3390/engproc2023055022
APA StyleZhu, N., Takeuchi, Y., Amano, K., & Fukuda, K. (2023). Development of Electric Power Generator by Using Hydrogen. Engineering Proceedings, 55(1), 22. https://doi.org/10.3390/engproc2023055022






