Abstract
Four process parameters were investigated via regression analysis for their effects on biodiesel production in the transesterification of biodiesel from waste soybean oil (WSO). The maximum biodiesel conversion rate reached 98.56% at a methanol/oil ratio of 6:1, a reaction time of 60 min, a reaction temperature of 60 °C, and a catalyst loading of 0.5 wt.%. The functional group was dominated by an ester with the wavenumber 1743.78, the group attribution C=O, a strong absorption intensity, and a stretching vibration. The major methyl ester compounds of the biodiesel yielded from the waste soybean oil were dominated by linoleic acid (37.53%), oleic acid (24.69%), and palmitic acid (12.95%). The regression analysis result showed that the parameters influenced the biodiesel yield, and that the reaction temperature contributed to the reaction more than the others.
1. Introduction
Biodiesel is one of the most promising fuels because its raw materials are abundant. Even though the cost of producing biodiesel is more than that of fossil fuels, many countries are producing biodiesel commercially to meet their energy needs because biodiesel assures energy security, environmental friendliness, and socio-economic stability. With similar properties to diesel oil, biodiesel can be used for any engine because of its excellent lubricity. It is a renewable resource (vegetable oil or animal fat) and is biodegradable and environmentally friendly. To produce biodiesel from vegetable oil, transesterification is process most used to form a mixture of fatty acid methyl esters (FAMEs). Oils change their chemical and physical properties if they are cooked many times. Waste or used cooking oil can be used to produce biodiesel. The use of used cooking oil as a raw material (feedstock) may increase the production of biodiesel and reduce environmental pollution [1]. Soybean oil is the second most consumed vegetable oil in the world; hence, used soybean oil can be used in many countries for the production of biodiesel [2,3,4,5].
Producing biodiesel from waste soybean oil via transesterification requires a catalyst. There are many kinds of catalysts, including base and acid catalysts, that can increase biodiesel production in the transesterification reaction. Catalysts must be chosen considering environmental aspects and costs. Potassium hydroxide, one of the base catalysts, is used in the transesterification reaction and has shown a conversion yield of ethyl or methyl ester of over 90% [1,6]. The transesterification reaction is shown in Equation (1).
Triglycerides are heavy molecular fats and oils saponified (hydrolyzed) in a solution to produce soap and glycerol. The glycerol is insoluble in the transesterification product and easily separated with methyl ester because of its higher density. In transesterification reactions, the soap is formed because free fatty acids (FFAs) from the oil react with the catalyst. It is a reactant in the transesterification containing water, and the reaction increases the quantity of FFAs in hydrolysis. This increases the formation of soap. The process of soap formation with a high FFA content is expressed as follows:
In transesterification, the most active catalysts are methoxide radicals. The activity of a particular catalyst depends on the number of methoxide radicals. These are used in the reaction, as each mole of triglyceride reacts with 3 moles of primary alcohol, and yields 3 moles of alkyl ester (biodiesel) and 1 mole of glycerol (by-product). Dissolving potassium in methanol produces the methoxide ion according to the following reaction:
OH− + 3CH3OH → 3CH3O− + H2O
Biodiesel purification using a conventional catalyst requires high energy and large water consumption, which causes environmental issues and affects the quality of the biodiesel. To increase the biodiesel quality, a purification process for removing impurities such as catalysts, methanol, glycerol, glyceride, water, soap, and other materials is necessary. There are two often used methods, namely wet washing and dry washing. The wet washing method is the best choice so far in purifying biodiesel after the transesterification reaction.
2. Materials and Methods
A transesterification method was used to obtain methyl ester biodiesel. In the transesterification and purification, the operating conditions and homogeneous mixture were controlled. The required raw materials were waste soybean oil, potassium hydroxide, and methanol. Soybean oil was purchased from a local supermarket in Tainan, Taiwan. It was used for frying fish or meat, and then became waste soybean oil as a feedstock. Methanol was chosen because it was more suitable than other alcohols for transesterification when considering the price and physical and chemical properties. Potassium hydroxide (KOH) or sodium hydroxide (NaOH) were recommended to produce methyl ester. However, considering the cost, KOH was chosen as it is cheaper than NaOH. The operating conditions, regarding the oil/methanol molar ratios, catalyst loading, reaction time, and reaction temperature, are presented in Table 1.

Table 1.
Operating conditions used to convert triglycerides from waste soybean oil to methyl ester biodiesel.
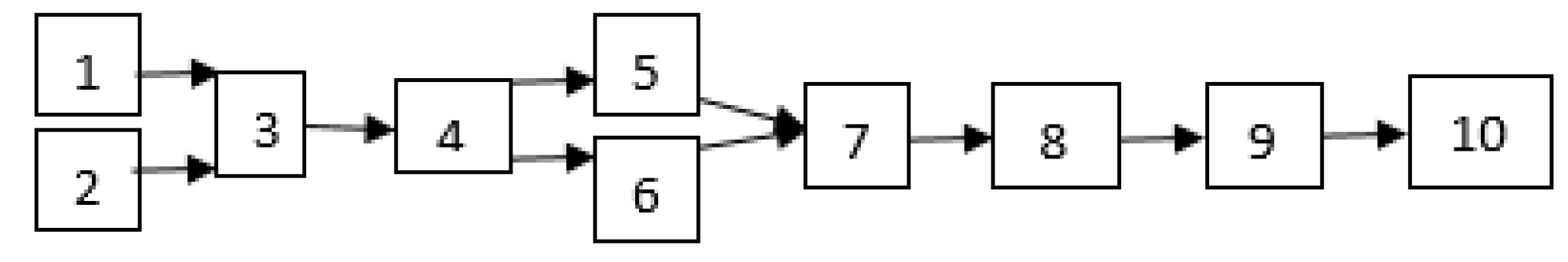
First, waste soybean oil was heated up to 40 °C and then filtered to remove dirt, charred food, and non-oil materials. Then, the oil was heated up to above 100 °C to remove the water content. The catalyst was added to the methanol and stirred until all KOH dissolved, and then it was mixed into the heated oil. The mixture was heated on a hot plate stirrer. After heating, the separation of methyl ester and glycerin was performed for 60 min in a separatory funnel. The glycerin waste was drawn off from the bottom of the settling vessel via gravity. The experimental process of biodiesel production is shown in Figure 1 and Figure 2.
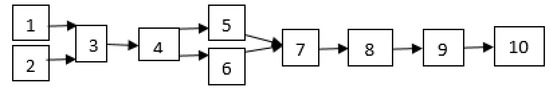
Figure 1.
Biodiesel production process; 1. Catalyst and methanol, 2. WSO, 3. Transesterification tank, 4. Separatory funnel, 5. Glycerol, 6. Biodiesel result of transesterification, 7. Purification/washing, 8. Heating, 9. Methyl ester biodiesel, 10. Physical and chemical property test.

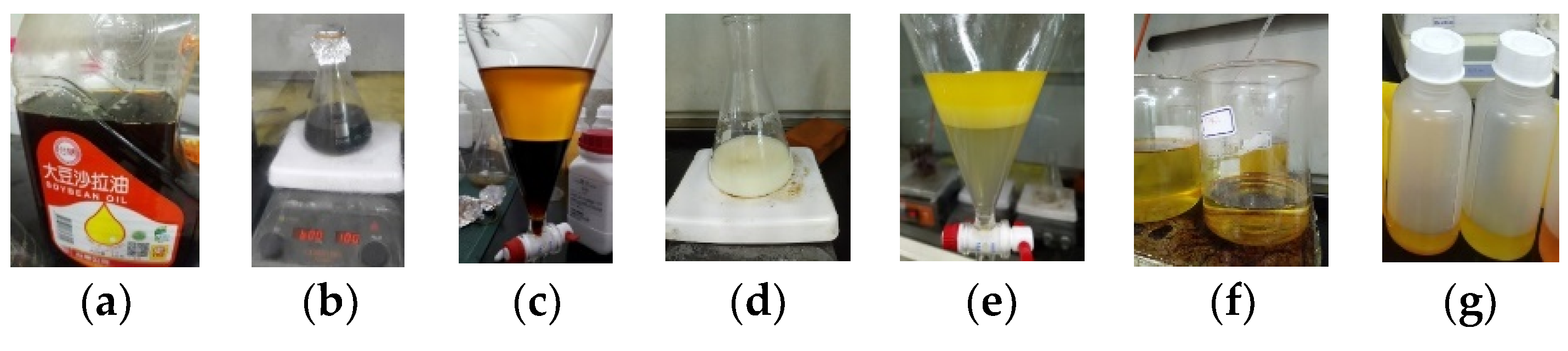
Figure 2.
Process of biodiesel production: (a) waste soybean oil (WSO); (b) transesterification reaction; (c) separation process between biodiesel and glycerol; (d) the washing process; (e) separation process between biodiesel and water; (f) the heating process; (g) pure biodiesel.
2.1. Analysis of Physicochemical Properties
The properties of biodiesel depend on the type and composition of the oil and the transesterification operating conditions. FT-IR spectroscopy was used to identify the presence of functional groups in the soybean oil, waste soybean oil, and biodiesel samples. Equation (4) was applied to calculate the yield of the obtained biodiesel.
Yield of biodiesel (%) = [(biodiesel (wt.))/(WCO (wt.))] × 100%
2.2. Regression Model
Regression analysis was used to analyze the experimental results. The variables are shown in Table 1. The F-test was conducted to determine the effects of the independent variable (X) on the dependent variable (Y) by comparing F-values at a significance level of 5%. By finding a partial coefficient, the fitness of the model was tested. The effect was identified by calculating the t-value and comparing it at a significance level of 5%. In the regression model, the parameters (Table 1) of eight reaction temperatures, five methanol/ oil ratios, six reaction times, and four catalyst loadings were included as independent variables, while biodiesel production (%) was included as a dependent variable. In the regression analysis, each independent variable’s effect on the dependent variable was identified, and function coefficients and polynomial functions were found. The regression equation was a polynomial function that depended on the degree of freedom. The polynomial model equation was as follows:
By entering all the variable conditions, the regression equation for eight reaction temperatures was derived as follows:
where Y is for the biodiesel conversion, an, a(n−1), a(n−2), …, a2, a1, a0 are function coefficients, n is the degree of freedom of the polynomial function, and X is the independent variable.
3. Results and Discussion
3.1. Regression Analysis
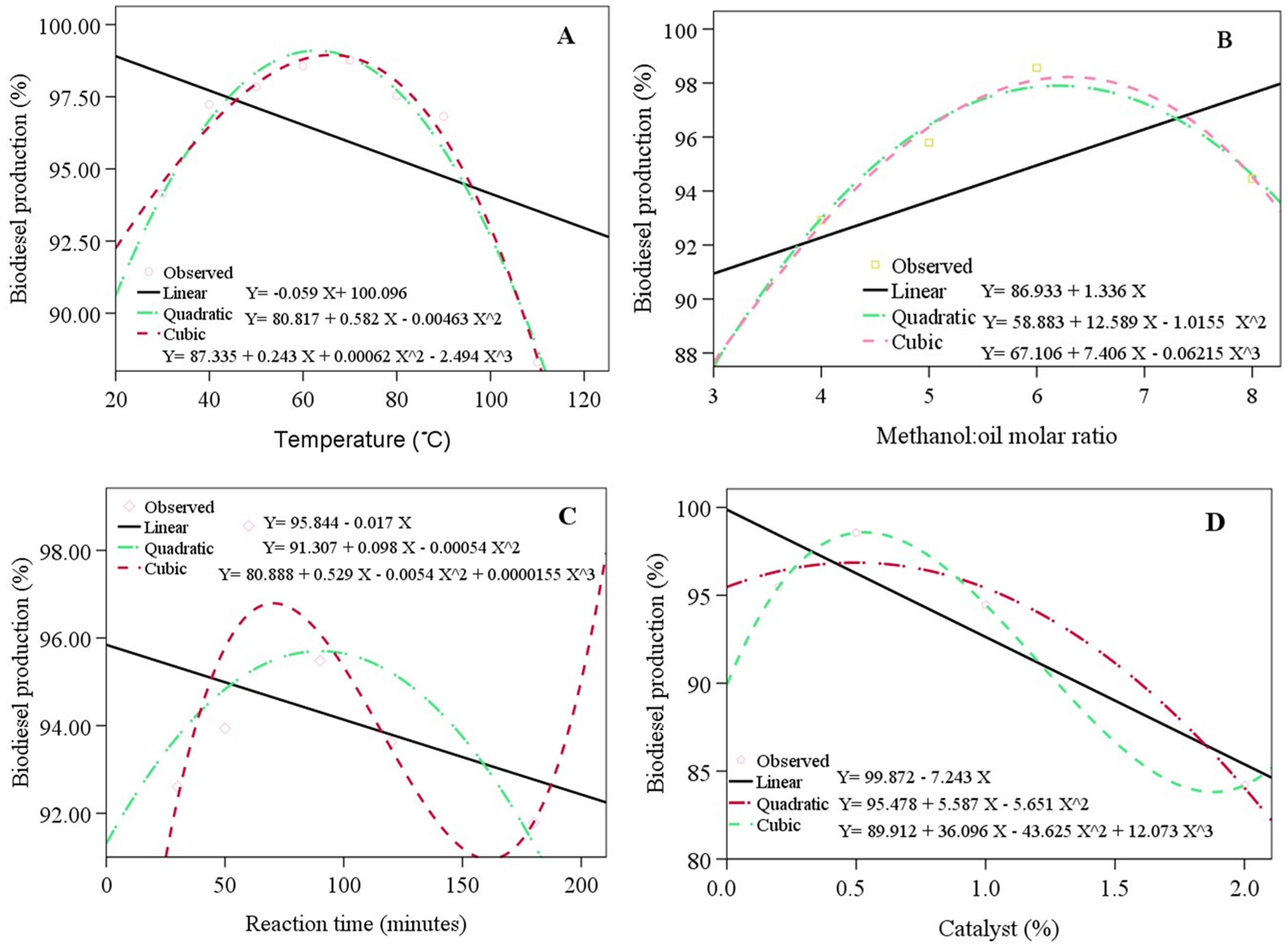
Figure 3 presents the estimation curves, which resemble quadratic and cubic curves. The coefficients of determination (R2) of the linear, quadratic, and cubic curves were 0.209, 0.972, and 0.982 (with the variation in reaction temperature), 0.405, 0.985, and 0.991 (with the variation in the methanol/oil ratio), 0.150, 0.450, and 0.729 (with the variation in reaction time), and 0.839, 0.960, and 1 (with the variation in catalyst loading). The cubic curve was similar to the observation data (R2 = 1) compared with the quadratic estimation curve for all the variations.
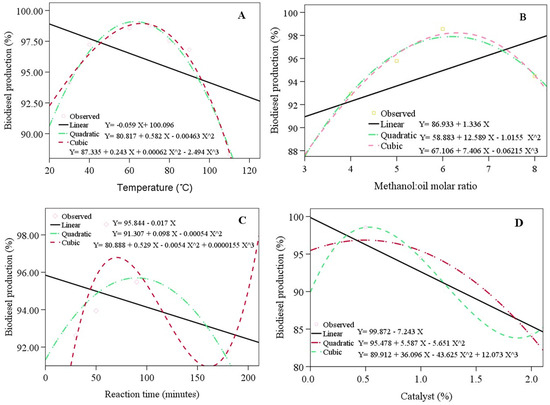
Figure 3.
The biodiesel production with the variations in the reaction temperature (A), methanol/oil ratio (B), reaction time (C), catalyst loading (D), the observation data compared with the estimation curves.
The parameters of the reaction temperature (quadratic = 0.00 and cubic = 0.001), methanol/oil ratio (quadratic = 0.015 and cubic = 0.009), and catalyst loading (Sig. = 0.000) had a significant effect on biodiesel conversion. On the other hand, the reaction time did not influence the biodiesel conversion significantly. The effect of the reaction temperature (XT), methanol/oil ratio (Xmo), reaction time (Xt), and catalyst loading (Xc) on biodiesel conversion Y((YT), (Ymo), (Yt), (Yc) and (YT)), respectively, was estimated using the following regression models:
3.2. Chemical Analysis of Biodiesel Using Fourier Transform Infrared (FTIR) Spectrum
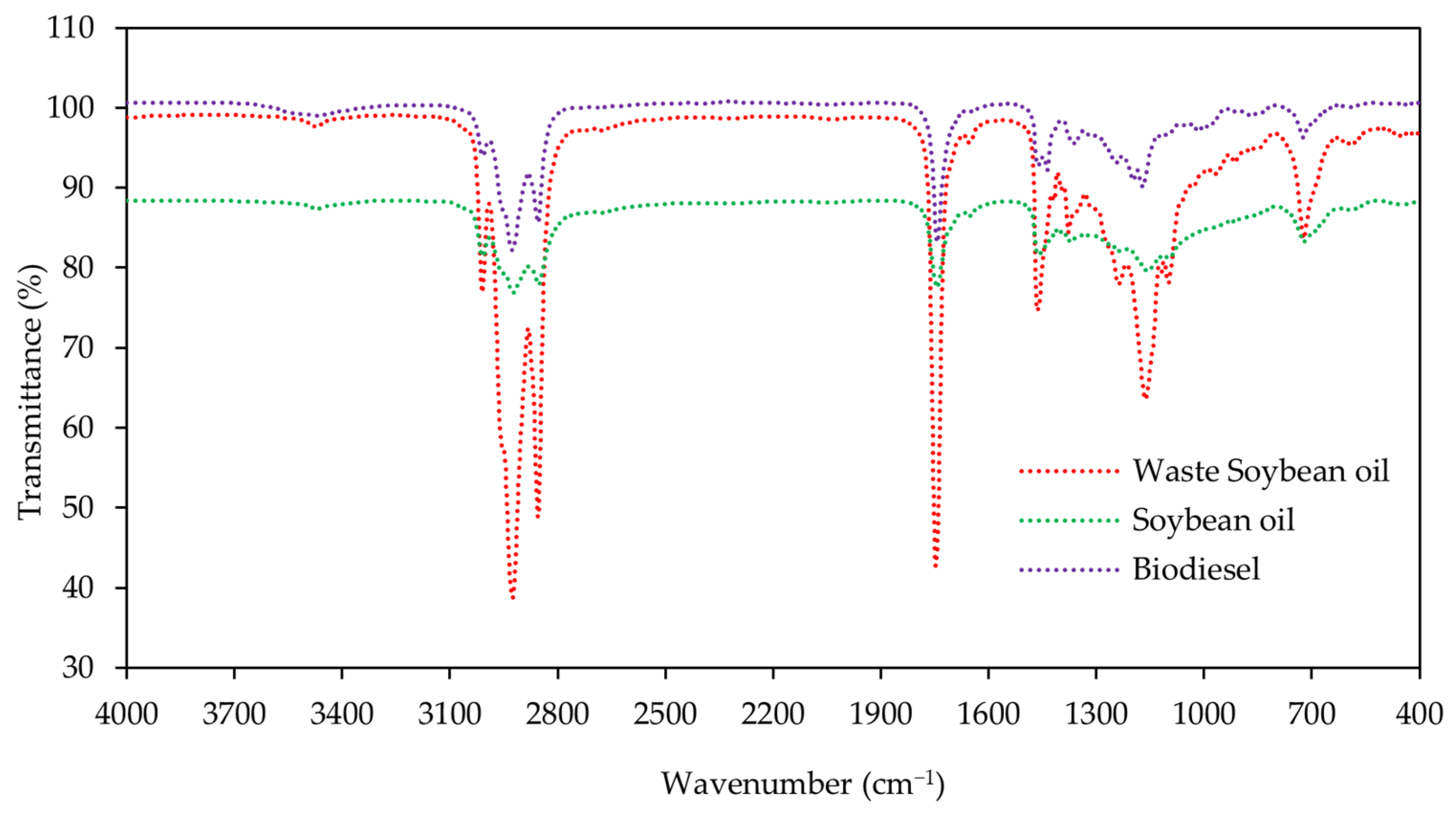
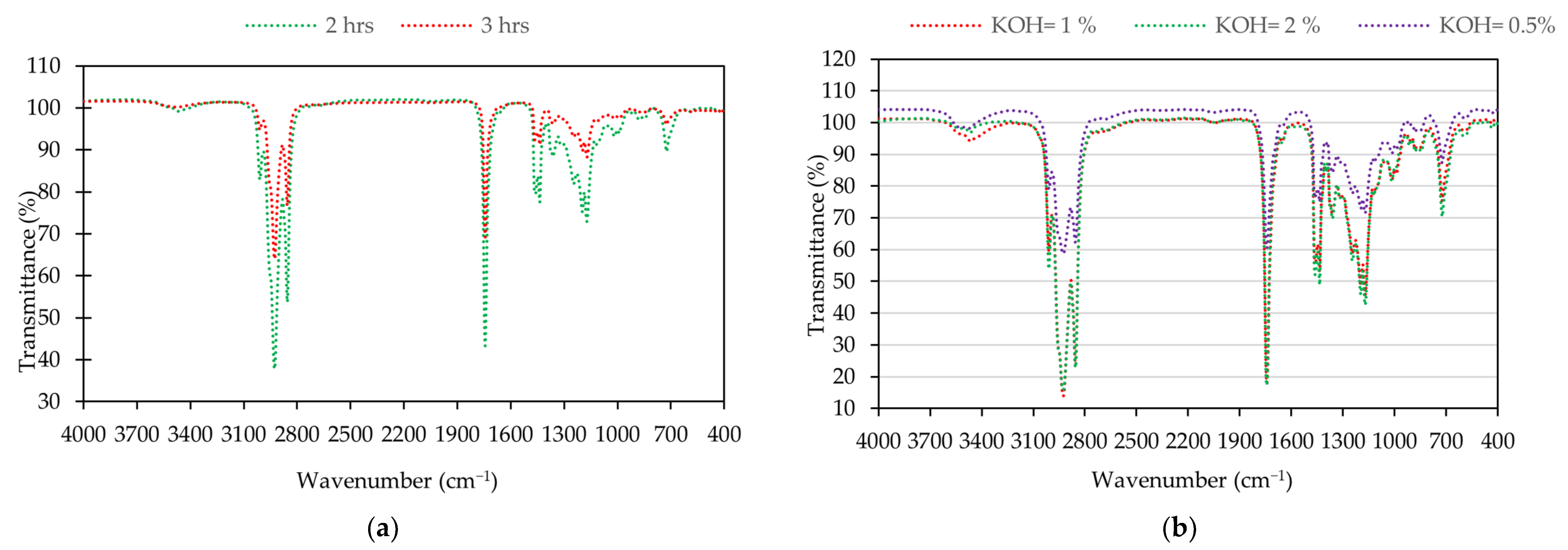
Figure 4 displays the FTIR spectra results for the main functional group of soybean oil, waste soybean oil, and biodiesel. Figure 5 shows the FTIR spectra of biodiesel under different reaction times and catalyst loadings, respectively. The spectra of the samples from soybean oil, waste soybean oil, and biodiesel were obtained at the ranges of 4000 to 400 cm−1.
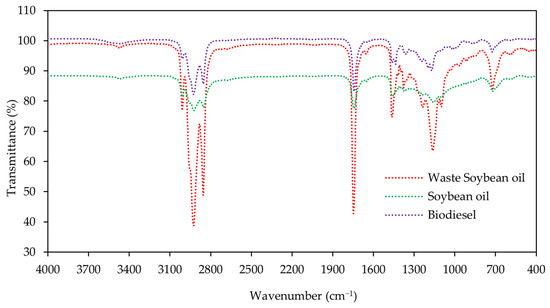
Figure 4.
Spectra at 4000–400 cm−1 were obtained for the soybean oil, waste soybean oil, and biodiesel using the FTIR technique.
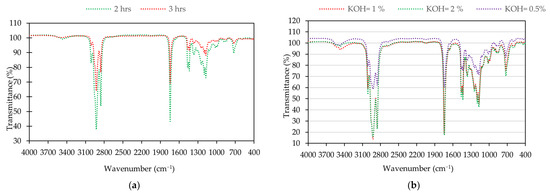
Figure 5.
Biodiesel: (a) FTIR spectra at the reaction time of 2 and 3 hours; (b) FTIR spectra under the different catalyst loadings.
There were no significant peaks in the regions of 1700−2800 cm−1 and 3000−4000 cm−1 for the soybean oil, waste soybean oil, and biodiesel for all conditions. There were strong peaks between 2800 cm−1 and 3000 cm−1, corresponding to the C-H vibration. The main characteristic peak for biodiesel was obtained at the spectrum of 1245 cm−1 (C-O), corresponding to the proportion of fatty acid methyl ester. Other peaks for biodiesel were identified at the spectra of 1744 cm−1 (C=O) and 1057 cm−1 (C-O).
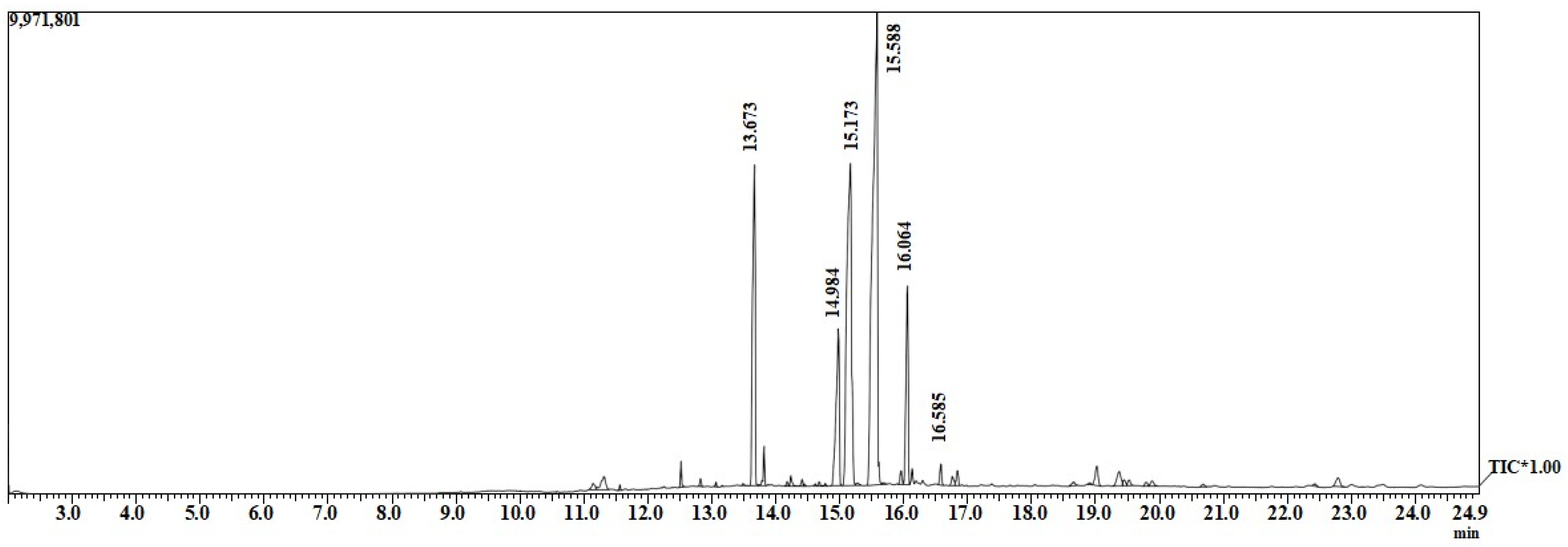
3.3. Gas Chromatography-Mass Spectrum (GC-MS) of Biodiesel Yield from Waste Soybean Oil
GC-MS was applied to determine the substance compositions or quality of the biodiesel in terms of the presence of free fatty acid methyl ester. Figure 6 shows the chromatography of biodiesel used to identify the compositions present in FAME. Table 2 shows data from the GC-MS results, indicating the peak number, methyl ester names, molecular weight, and approximate area values for each of the methyl ester compositions. The dominant fatty acids were linoleic acid (37.53%), followed by oleic acid (24.69%) and palmitic acid (12.95%).
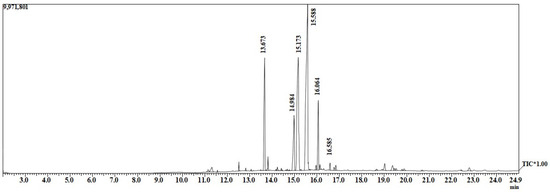
Figure 6.
GC-MS of biodiesel made of waste soybean oil.

Table 2.
Contents of methyl ester in the biodiesel yielded from waste soybean oil using GC-MS.
4. Conclusions
The transesterification process produced biodiesel by reacting triglycerides with used soybean oil with methanol and a KOH catalyst. Through the analysis of regression with four process parameters, the maximum methyl ester biodiesel produced from waste soybean oil was predicted at the operating conditions of 6:1 (methanol/ oil ratio), 0.5% (KOH catalyst), 60 min (reaction time), 60 °C (reaction temperature), and 600 rpm (stirrer speed). The reaction temperature, methanol to oil ratio, reaction time, and catalyst loading to the biodiesel conversion formed a cubic curve, which was fitted in the regression analysis. The FTIR spectra of the ester (C=O) were found at 1743, 1746, and 1743 cm−1 for the soybean oil, waste soybean oil, and biodiesel, respectively. The prominent characteristic peaks for biodiesel were identified at the spectra of 1245 cm−1 (C-O) and 1057 cm−1 (C-O). The GC-MS result showed that the major methyl ester components of the biodiesel yielded from waste soybean oil were linoleic acid (37.53%), followed by oleic acid (24.69%) and palmitic acid (12.95%).
Author Contributions
Conceptualization, M.; methodology, M. and W.-C.C.; software, M.; validation, M. and W.-C.C.; experiment data capture, M.; formal analysis, M.; writing—original draft preparation, M.; writing—review and editing, W.-C.C.; supervision, W.-C.C.; funding acquisition, W.-C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Science and Technology Council, Taiwan, grant number NSTC 111-2221-E-218-010.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data described in this study will be provided upon request.
Acknowledgments
The authors would like to express special thanks to the Instrument Equipment Division, Core Facility Center, NCKU, for providing FTIR and GC-MS to complete the important tests of biodiesel.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ejikeme, P.M.; Anyaogu, I.D.; Ejikeme, C.L.; Nwafor, N.P.; Egbuonu, C.A.; Ukogu, K.; Ibemesi, J.A. Catalysis in Biodiesel Production by Transesterification Processes-An Insight. J. Chem. 2010, 4, 1120–1132. [Google Scholar] [CrossRef]
- Rajesh, K.; Natarajan, M.P.; Devan, P.K.; Ponnuvel, S. Coconut fatty acid distillate as novel feedstock for biodiesel production and its characterization as a fuel for diesel engine. Renew. Energy 2021, 164, 1424–1435. [Google Scholar] [CrossRef]
- Karthikeyan, M. Production of biodiesel from Cordiamyxa bio-oil using BaMoO4 -Ce2O3 nanoparticles as an alternative fuel for diesel engine. Mater. Lett. 2019, 243, 199–201. [Google Scholar] [CrossRef]
- Gónzalez, J.P.C.; Gutiérrez, P.E.Á.; Medina, M.A.; Zapata, B.Y.L.; Guerrero, G.V.R.; Valdés, L.G.V. Effects on biodiesel production caused by feed oil changes in a continuous stirred-tank reactor. Appl. Sci. 2020, 10, 992. [Google Scholar] [CrossRef]
- García-Martín, J.F.; Barrios, C.C.; Alés-Álvarez, F.J.; Dominguez-Sáez, A.; lvarez-Mateos, P.A. Biodiesel production from waste cooking oil in an oscillatory flow reactor: Performance as a fuel on a TDI diesel engine. Renew. Energy 2018, 125, 546–556. [Google Scholar] [CrossRef]
- Blinová, L.; Fiala, J.; Balog, K. Biodiesel Production from Waste Cooking Oil in Laboratory Scale. Renew. Energy Environ. Technol. 2014, 448, 1656–1659. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).