Abstract
The aim of this study was to investigate some chemical and microbiological characteristics of the soil and the surface biofilms of both polypropylene microplastics and quartz sand. The exposure of sterile samples lasted for 30 days in the soil of a residential area. Some initial chemical and microbiological characteristics of the soil were studied, as well as microbiological characteristics of the biofilms on the surface of the materials. This expands the understanding of biofilm formation processes on the surface of microplastics in soil and can be used in processes for removing harmful materials from soil.
1. Introduction
Microplastics are defined as synthetic organic polymer particles with a size (or, more precisely, a maximum size) <5 mm [1]. The problem of environmental pollution caused by microplastic accumulation in various ecosystems draws attention from the whole world [1,2,3,4] and Ukraine specifically [5,6].
The biological activity of microorganisms on polymeric materials that enter the soil, in particular as garbage, leads to biofilm formation and initialization of material biodegradation occurs [7,8]. Among soil microorganisms, as biodegraders of synthetic polymers, heterotrophic bacteria and sulfate-reducing bacteria (SRB) deserve attention since they are part of the plastisphere and are able to form a biofilm on the surfaces of plastics [8,9,10,11,12]. The aim of this study was to investigate some chemical and microbiological characteristics of the soil and the surface biofilms of both polypropylene microplastics and quartz sand.
2. Materials and Methods
Particles of quartz sand (Poland) and primary pellets of polypropylene microplastics (Republic of Serbia) with a size of 3–5 mm in the amount of 15 pieces for each of the three repetitions of each variant were used for the research. The mass of the 15 samples in each case was determined by weighing in order to determine the volume of materials used in the study. Before the start of the experiment, the biofilm was removed from the samples by ultrasound [13] and the samples were sterilized with 70% ethyl alcohol, which was removed by washing the samples in sterile distilled water before applying them to the soil. The samples were mixed with the soil in sterile polypropylene containers, each of 120 mL and with 5 holes of 1 mm diameter at the bottom. Open containers were placed in the soil at a depth of 20–25 cm.
The exposure of samples lasted for 30 days in the sod–podzolic soil of a residential area (which was not subjected to anthropogenic influence) in Chernihiv, Ukraine (51°29′58″ N, 31°16′08″ E). Some initial chemical characteristics of the soil (nitrate nitrogen, ammonium nitrogen, pH, sulfates, chlorides, sulfur, humidity) were studied in the laboratory of the Government Agency “Chernihiv Regional Center for Disease Control and Prevention” of the Ministry of Health of Ukraine (Chernihiv, Ukraine) following the methods recommended by regulatory documents of Ukraine. A soil sample selected by a generally accepted method was used for the study [14]. Microbiological characteristics of the soil and the biofilms on the surface of the materials were studied in the laboratory of T.H. Shevchenko National University “Chernihiv Colehium” (Chernihiv, Ukraine): the total microbial count (TMC)—deep inoculation of soil suspension dilutions (or dilutions of a physiological solution of NaCl with biofilm removed by ultrasound) in meat-peptone agar, aerobic cultivation conditions at 37 °C; the number of sulfate-reducing bacteria—inoculation of dilutions of soil suspension (or dilutions of a physiological solution of NaCl with biofilm removed by ultrasound) into liquid Postgate’s “C” medium, anaerobic cultivation conditions at 29 ± 2 °C. The preparation of the soil suspension and its dilutions was carried out according to the generally accepted method [15]. Samples of the materials were taken from the soil, washed with a sterile physiological solution of NaCl from the cells of microorganisms that did not attach to the surface, and then the biofilm was removed from the surface to determine the number of the indicated groups of microorganisms. The biofilms on the surfaces of quartz sand particles and polypropylene microplastics (pre-production pellets) were removed in a physiological NaCl solution by ultrasound (28 kHz) as described earlier [16].
When analyzing the results, statistical methods were used: the number of bacteria in the liquid medium was determined using McCready tables, and the number of bacteria in a dense medium was determined by calculating the standard error of the arithmetic mean.
3. Results and Discussion
The values of investigated chemical characteristics are within the limits of normative characteristics (Table 1).

Table 1.
Chemical characteristics of the studied soil.
It was established that the studied soil was uncontaminated in terms of sanitary–hygienic and microbiological safety—the TMC was 1.26 ± 0.15 × 105 colony forming units (CFU)/1 g abs. dry soil and did not exceed the normative indicator 5 × 105 CFU/1 g abs. dry soil [14].
The number of SRB was 2.61 × 104 cells/1 g abs. dry soil, which matches the characteristics of natural soil [17]. According to the TMC and the number of SRB in the studied soil, it can be suggested that it has a sufficiently high biological activity; the studied soil can be used to study the effects of microplastic accumulation in the environment.
Examples of samples of the studied materials removed from the soil and washed with sterile physiological NaCl solution are presented in Figure 1.
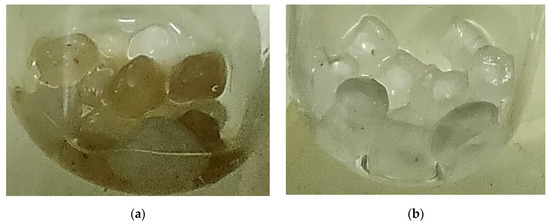
Figure 1.
The samples of materials removed from the soil and washed with sterile physiological NaCl solution: (a) quartz sand and (b) polypropylene microplastic.
In the biofilms of quartz sand and polypropylene, the TMC was at the same level—1.59 ± 0.27 × 103 and 1.55 ± 0.19 × 103 CFU/cm3, respectively. The numbers of SRB were 1.24 × 104 and 2.11 × 104 cells/cm3, respectively, in the biofilms of quartz sand and polypropylene. Thus, the number of SRB in the biofilm on the surface of polypropylene was 1.7 times higher than in the biofilm on the surface of quartz sand. In this case, the obtained data are consistent with the results of Rogers et al. [18] regarding the greater number of microorganisms on plastic than on the surface of a material such as copper. It has also been shown that small and large polyethylene particles have significantly higher levels of bacteria than glass [19]. Actinobacteria, Proteobacteria, and Patescibacteria have been established as the main microbiome of the “plastisphere” of polypropylene and expanded polystyrene [7].
Chen et al. [8] established that the biomass of the biofilm is higher on the 30th day than on the 15th day. In general, inhabitation of the microplastic surface by microorganisms occurs within hours, and on the 14th day, the biofilm is fully formed [20]. The content of extracellular polymers in biofilms on microplastics also increases over time [8]. Microorganisms that concentrate on the surface of microplastics can biodegrade organic pollutants and degrade organic compounds, and they play a significant role in the global carbon cycle and climate regulation. In addition, microplastics can affect the functioning of the soil microbial community [8].
4. Conclusions
The obtained results expand the understanding of biofilm formation processes on the surface of microplastics in soil and the participation of microorganisms in their biodegradation. It can be used in processes for removing harmful materials from soil.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ASEC2023-15350/s1, Conference Presentation: Some microbiological characteristics of the biofilm on the surface of pre-production pellets of polypropylene microplastics after short exposure in the soil.
Author Contributions
Conceptualization, N.T. and L.Z.; Methodology, N.T.; Validation, N.T.; Formal Analysis, N.T.; Investigation, N.T.; Resources, N.T.; Writing—Original Draft Preparation, N.T. and L.Z.; Writing—Review and Editing, N.T. and L.Z.; Visualization, N.T.; Supervision, N.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used in this research are available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Duis, K.; Coors, A. Microplastics in the aquatic and terrestrial environment: Sources (with a specific focus on personal care products), fate and effects. Environ. Sci. Eur. 2016, 28, 2. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.K.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef] [PubMed]
- Chia, R.W.; Lee, J.Y.; Lee, M.; Lee, G.S.; Jeong, C.D. Role of soil microplastic pollution in climate change. Sci. Total. Environ. 2023, 887, 164112. [Google Scholar] [CrossRef] [PubMed]
- Dike, S.; Apte, S. Impact of microplastic pollution in terrestrial ecosystem on index and engineering properties of sandy soil: An experimental investigation. Sci. Total. Environ. 2023, 887, 164049. [Google Scholar] [CrossRef] [PubMed]
- Yurchenko, V.O.; Melnikova, O.G.; Ponomaryov, K.S.; Samokhvalova, A.I. Microplastics in bottom sediments of rivers in urbanized areas. In Proceedings of the International Scientific and Practical Internet Conference “Ecologically Sustainable Development of Urban Systems: Challenges and Solutions” KhNUUE named after O. M. Beketova, Kharkiv, Ukraine, 2–3 November 2021; Available online: http://eprints.kname.edu.ua/60576/1/C%D0%B1%D0%BE%D1%80%D0%BD%D0%B8%D0%BA21-134-136.pdf (accessed on 26 June 2023). (In Ukrainian).
- Fortuna, M.; Borysovska, O. Assessment of water pollution by microplastic. Collect. Sci. Work. Natl. Min. Univ. 2021, 65, 195–206. Available online: http://znp.nmu.org.ua/index.php/en/archives/37-65en/441-65en18 (accessed on 26 June 2023). (In Ukrainian). [CrossRef]
- Kublik, S.; Gschwendtner, S.; Magritsch, T.; Radl, V.; Rillig, M.C.; Schloter, M. Microplastics in soil induce a new microbial habitat, with consequences for bulk soil microbiomes. Front. Environ. Sci. 2022, 10, 989267. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Wang, X.; Cheng, T.; Fu, K.; Qin, Z.; Feng, K. Biofilm Structural and Functional Features on Microplastic Surfaces in Greenhouse Agricultural Soil. Sustainability 2022, 14, 7024. [Google Scholar] [CrossRef]
- McCormick, A.; Hoellein, T.J.; Mason, S.A.; Schluep, J.; Kelly, J.J. Microplastic is an abundant and distinct microbial habitat in an urban river. Environ. Sci. Technol. 2014, 48, 11863–11871. [Google Scholar] [CrossRef] [PubMed]
- Tagg, A.S.; Oberbeckmann, S.; Fischer, D.; Kreikemeyer, B. Paint particles are distinct and variable substrate for marine bacteria. Mar. Pollut. Bull. 2019, 146, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Tkachuk, N.; Zelena, L.; Lukash, O.; Mazur, P. Microbiological and genetic characteristics of Bacillus velezensis bacillibactin-producing strains and their effect on the sulfate-reducing bacteria biofilms on the poly(ethylene terephthalate) surface. EQ 2021, 32, 119–129. [Google Scholar] [CrossRef]
- Tkachuk, N.; Zelena, L. The Impact of Bacteria of the Genus Bacillus upon the Biodamage/Biodegradation of Some Metals and Extensively Used Petroleum-Based Plastics. Corros. Mater. Degrad. 2021, 2, 531–553. [Google Scholar] [CrossRef]
- Yao, Y.; Rao, S.; Habimana, O. Active Microbiome Structure and Functional Analyses of Freshwater Benthic Biofilm Samples Influenced by RNA Extraction Methods. Front. Microbiol. 2021, 12, 588025. [Google Scholar] [CrossRef] [PubMed]
- Yeromina, A.K.; Goncharova, N.G.; Sokolovska, I.A. Ecology of Microorganisms: Teaching. Manual for Students of the 3rd Year of the Medical Faculty, Specialty “Laboratory diagnostics”; ZSMU: Zaporizhzhia, Ukraine, 2013; 75p, Available online: http://library.zsmu.edu.ua/cgi/irbis64r_14/fulltext/Rejting/Er'ominaAK13_Ekolo_m.pdf (accessed on 26 June 2023). (In Ukrainian)
- Furzikova, T.M.; Serhiychuk, M.G.; Vlasenko, V.V.; Shvets, Y.u.V.; Pozur, V.K. Microbiology. Practicum; Phytosocial Center: Kyiv, Ukraine, 2006; 210p. (In Ukrainian) [Google Scholar]
- Tkachuk, N.; Zelena, L. Bacterial sulfidogenic community from the surface of technogenic materials in vitro: Composition and biofilm formation. Biofouling 2023, 39, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Abdulina, D.R.; Asaulenko, L.G.; Purish, L.M. Diversity of corrosive aggressive bacteria in soils of different biotopes. Stud. Biol. 2011, 5, 11–16. [Google Scholar] [CrossRef][Green Version]
- Rogers, J.; Dowsett, A.B.; Dennis, P.J.; Lee, J.V.; Keevil, C.W. Influence of plumbing materials on biofilm formation and growth of Legionella pneumophila in potable water systems. Appl. Environ. Microbiol. 1994, 60, 1842–1851. [Google Scholar] [CrossRef]
- Parrish, K.; Fahrenfeld, N.L. Microplastic biofilm in fresh- and wastewater as a function of microparticle type and size class. Environ. Sci. Water Res. Technol. 2019, 5, 495–505. [Google Scholar] [CrossRef]
- Harrison, J.P.; Schratzberger, M.; Sapp, M.; Osborn, A.M. Rapid bacterial colonization of low-density polyethylene microplastics in coastal sediment microcosms. BMC Microbiol. 2014, 14, 232. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).