Evaluation of the Potential of Microalgae as Bioremediation Agents for Olive Mill Wastewater †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalgae
2.2. Wastewaters
2.3. Experimental Setup
2.4. Analytical Determinations
3. Results and Discussion
3.1. Wastewater Compositions
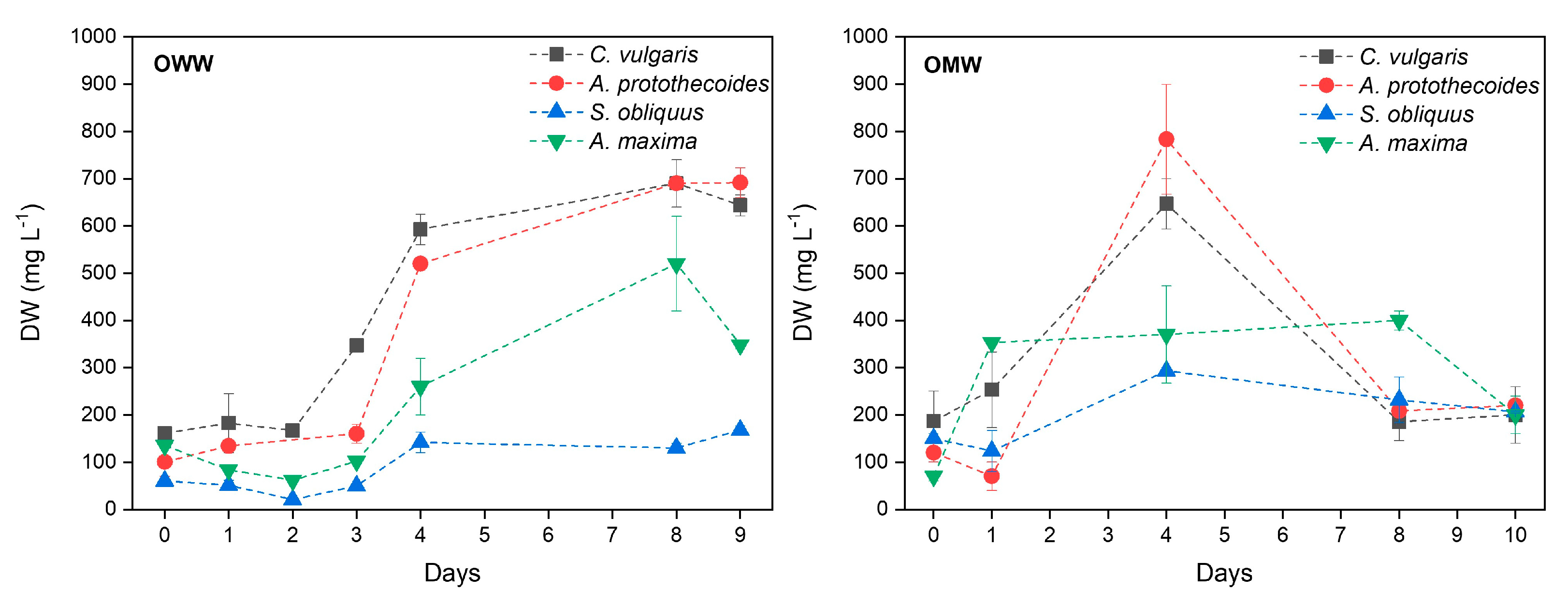
3.2. Microalgal Growth
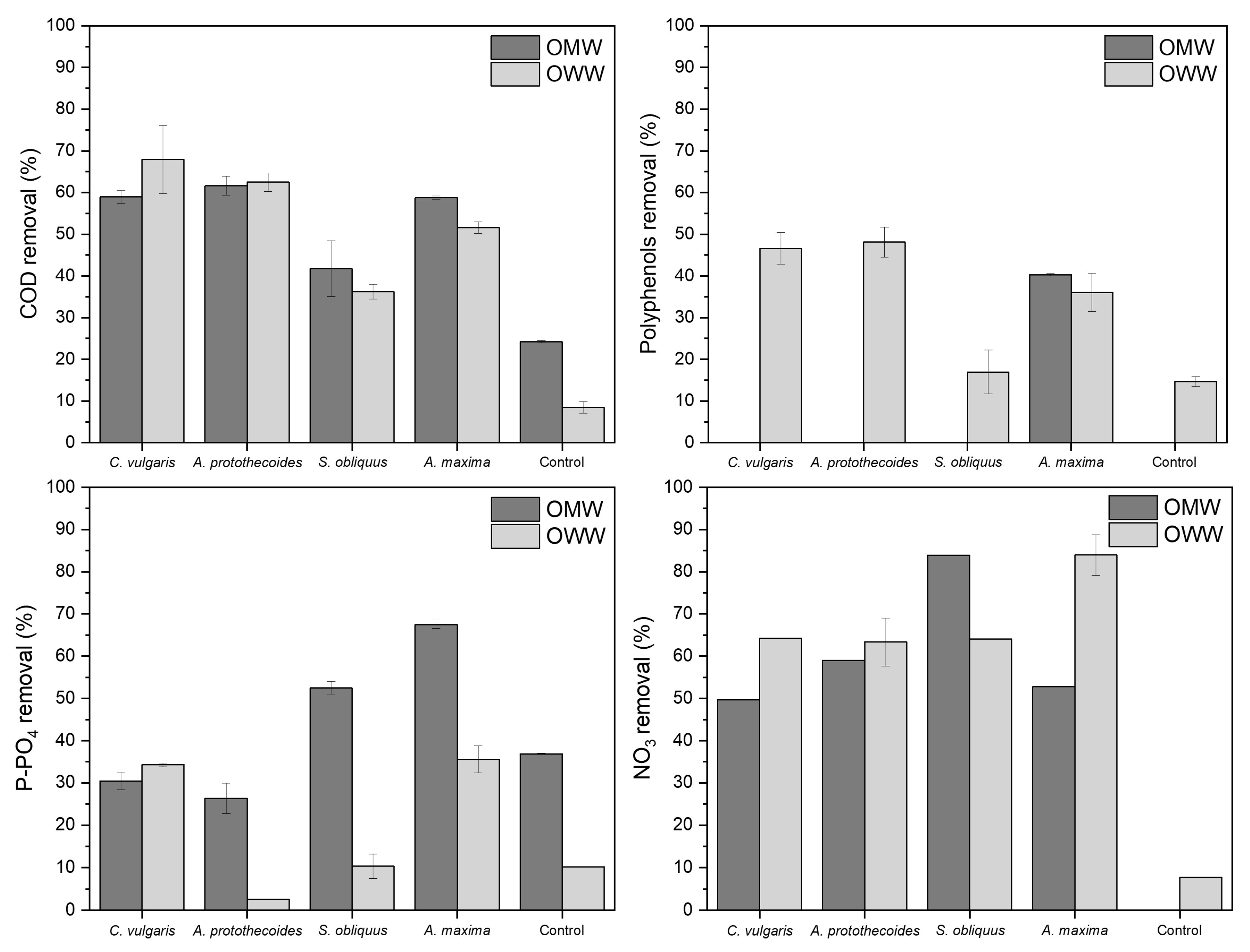
3.3. Bioremediation Potential
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amor, C.; Marchão, L.; Lucas, M.S.; Peres, J.A. Application of Advanced Oxidation Processes for the Treatment of Recalcitrant Agro-Industrial Wastewater: A Review. Water 2019, 11, 205. [Google Scholar] [CrossRef]
- Ioannou-Ttofa, L.; Michael-Kordatou, I.; Fattas, S.C.; Eusebio, A.; Ribeiro, B.; Rusan, M.; Amer, A.R.B.; Zuraiqi, S.; Waismand, M.; Linder, C.; et al. Treatment efficiency and economic feasibility of biological oxidation, membrane filtration and separation processes, and advanced oxidation for the purification and valorization of olive mill wastewater. Water Res. 2017, 114, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Marchão, L.; Fernandes, J.R.; Sampaio, A.; Peres, J.A.; Tavares, P.B.; Lucas, M.S. Microalgae and immobilized TiO2/UV-A LEDs as a sustainable alternative for winery wastewater treatment. Water Res. 2021, 203, 117464. [Google Scholar] [CrossRef]
- Hodaifa, G.; Malvis, A.; Maaitah, M. Combination of physicochemical operations and algal culture as a new bioprocess for olive mill wastewater treatment. Biomass Bioenergy 2020, 138, 105603. [Google Scholar] [CrossRef]
- Malvis, A.; Hodaifa, G.; Halioui, M.; Seyedsalehi, M.; Sánchez, S. Integrated process for olive oil mill wastewater treatment and its revalorization through the generation of high added value algal biomass. Water Res. 2019, 151, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Maaitah, M.; Hodaifa, G.; Malvis, A.; Sánchez, S. Kinetic growth and biochemical composition variability of Chlorella pyrenoidosa in olive oil washing wastewater cultures enriched with urban wastewater. J. Water Process Eng. 2020, 35, 101197. [Google Scholar] [CrossRef]
- Vonshak, A. Laboratory techniques for the cultivation of microalgae. In CRC Handbook of Microalgal Mass; Culture, R.A., Ed.; CRC Press: Boca Raton, FL, USA, 1986; pp. 117–143. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998; Volume 51. [Google Scholar] [CrossRef]
- Comissão Técnica C 720/CT 72. Determinação de Nitratos. Parte 1: Método Espectrométrico do 2,6 Dimetilfenol. NP 4338-1, 1st ed.; Instituto Português da Qualidade: Monte da Caparica, Portugal, 1996. [Google Scholar]
- Hillebrand, H.; Sommer, U. The nutrient stoichiometry of benthic microalgal growth: Redfield are optimal proportions. Limnol Ocean. 1999, 44, 440–446. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Lindner, A.V.; Pleissner, D. Utilization of phenolic compounds by microalgae. Algal Res. 2019, 42, 101602. [Google Scholar] [CrossRef]
- Gonçalves, A.L.; Pires, J.C.M.; Simões, M. A review on the use of microalgal consortia for wastewater treatment. Algal Res. 2017, 24, 403–415. [Google Scholar] [CrossRef]
| Parameter | OMW | OWW |
|---|---|---|
| pH | 5.1 ± 0.1 | 4.1 ± 0.1 |
| EC (µS cm−1) | 270 ± 50 | 357 ± 12 |
| Turbidity (NTU) | 693 | 138 |
| TSS (mg L−1) | 699 | 118 |
| TOC (mg C L−1) | 67,130 | 2382 |
| TN (mg N L−1) | 809.9 | 33.3 |
| COD (mg O2 L−1) | 206,880 ± 1332 | 7789 ± 356 |
| BOD5 (mg O2 L−1) | 6050 ± 50 | 80 ± 10 |
| Polyphenols (mg gallic acid L−1) | 3875 ± 20 | 326 ± 69 |
| P-PO4 (mg P L−1) | 487 ± 6 | 18 ± 3 |
| NO3 (mg L−1) | 548 ± 21 | 49 ± 4 |
| Wastewater | Microalgae | PX, max (mg L−1 day−1) |
|---|---|---|
| OWW | C. vulgaris | 107.9 ± 15.3 |
| A. protothecoides | 73.7 ± 3.6 | |
| S. obliquus | 20.4 ± 7.6 | |
| A. maxima | 48.1 ± 16.8 | |
| OMW | C. vulgaris | 115.1 ± 18.9 |
| A. protothecoides | 165.8 ± 34.1 | |
| S. obliquus | 38.3 ± 4.2 | |
| A. maxima | 143.3 ± 22.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchão, L.; Teixeira, O.; Peres, J.A.; Tavares, P.B.; Lucas, M.S. Evaluation of the Potential of Microalgae as Bioremediation Agents for Olive Mill Wastewater. Eng. Proc. 2023, 56, 211. https://doi.org/10.3390/ASEC2023-15236
Marchão L, Teixeira O, Peres JA, Tavares PB, Lucas MS. Evaluation of the Potential of Microalgae as Bioremediation Agents for Olive Mill Wastewater. Engineering Proceedings. 2023; 56(1):211. https://doi.org/10.3390/ASEC2023-15236
Chicago/Turabian StyleMarchão, Leonilde, Olga Teixeira, José A. Peres, Pedro B. Tavares, and Marco S. Lucas. 2023. "Evaluation of the Potential of Microalgae as Bioremediation Agents for Olive Mill Wastewater" Engineering Proceedings 56, no. 1: 211. https://doi.org/10.3390/ASEC2023-15236







