Abstract
The mercury content is among the parameters that characterise the quality of Solid Recovered Fuels (SRFs), which constitute alternative solid fuels of increasing interest. In this study, a direct mercury analyser was utilised in the analyses of SRF samples originating from Automotive Shredded Residues (ASRs). Two SRFs and one liquid reference material were measured to test the accuracy and sensitivity of the instrument. The mean values of the two SRFs and one liquid reference material (RM) were 0.171, 0.324 mg/kg, and 0.141 mg/L. These values were compared with the reference ones as follows: 0.168, 0.382 mg/kg, and 0.137 mg/L. Mercury content was detected in the analysis samples of four different particle sizes (8 mm, 2 mm, 1 mm, 0.5 mm, and 0.25 mm) for each SRF sample in order to demonstrate the direct mercury analyser operation and to compare it as an alternative to mercury determination via atomic absorption spectrometry. The measurements showed that grinding down to 1 mm was sufficient for most SRF cases while grinding down to 2 mm might be enough for a few cases. As an overall conclusion, the direct mercury analyser can be regarded as an efficient laboratory tool, offering a robust alternative to the atomic absorption spectrometry procedure, especially in terms of accuracy, speed, safety, and cost-effectiveness.
1. Introduction
Solid Recovered Fuels (SRFs) refer to quality solid fuels derived from non-recyclable waste materials. They are produced from the non-biological dry fraction of waste streams, which are mainly those materials that cannot be recycled in an economically viable way. Automotive Shredded Residues (ASRs) may be considered Solid Recovered Fuels (SRFs) provided that all hazardous materials are removed since the main requirement for solids to be considered SRFs is not to contain hazardous substances, according to ISO 21640 [1]. Mercury may be present in exhaust gases due to its high vapor tension. It has been identified as one of the world’s most important environmental pollutants [2]. Recovering materials and energy from waste remains a priority among European policies since they promote sustainable growth and the circular economy [3]. ASR results from the shredding of End-of-Life Vehicles (ELVs) and is a by-product of the vehicle recycling process. ASR material is obtained by previously removing many recyclable vehicle parts, such as bumpers, airbags, batteries, fuel tanks, tires, and seats. ASR is a very heterogeneous material consisting of plastics, elastomers, glass, wood, paper, leather, textiles, sand, and metals [4]. Vigano et al. [5] report that the range of mercury in ASR is between 0 and 0.5 mg/kg on a dry basis.
The analytical challenges presented by measuring mercury using techniques like cold vapor atomic absorption/atomic fluorescence (CVAA/AF) or Inductively Coupled Plasma–Optical Emission or Mass Spectrometry (ICP/OES-MS) are well recognised. The inherent problem lies in the fact that all these techniques are solution-based, which means that if the sample is not a liquid, it has to be digested before it is introduced to the instrument. Moreover, when heterogeneous samples are analysed containing difficult-to-digest particles, it is necessary to grind them down to smaller particle sizes than those suggested by standard methods. Such a reduction in size is not an easy task when it comes to mixtures of plastic, elastomers, fibres, and leather, such as SRF samples. Thus, the risk of less representative analysis samples becomes significant. Therefore, the utilisation of equipment requiring less elaborate sample pretreatments is a good option, provided it presents an acceptable accuracy of results.
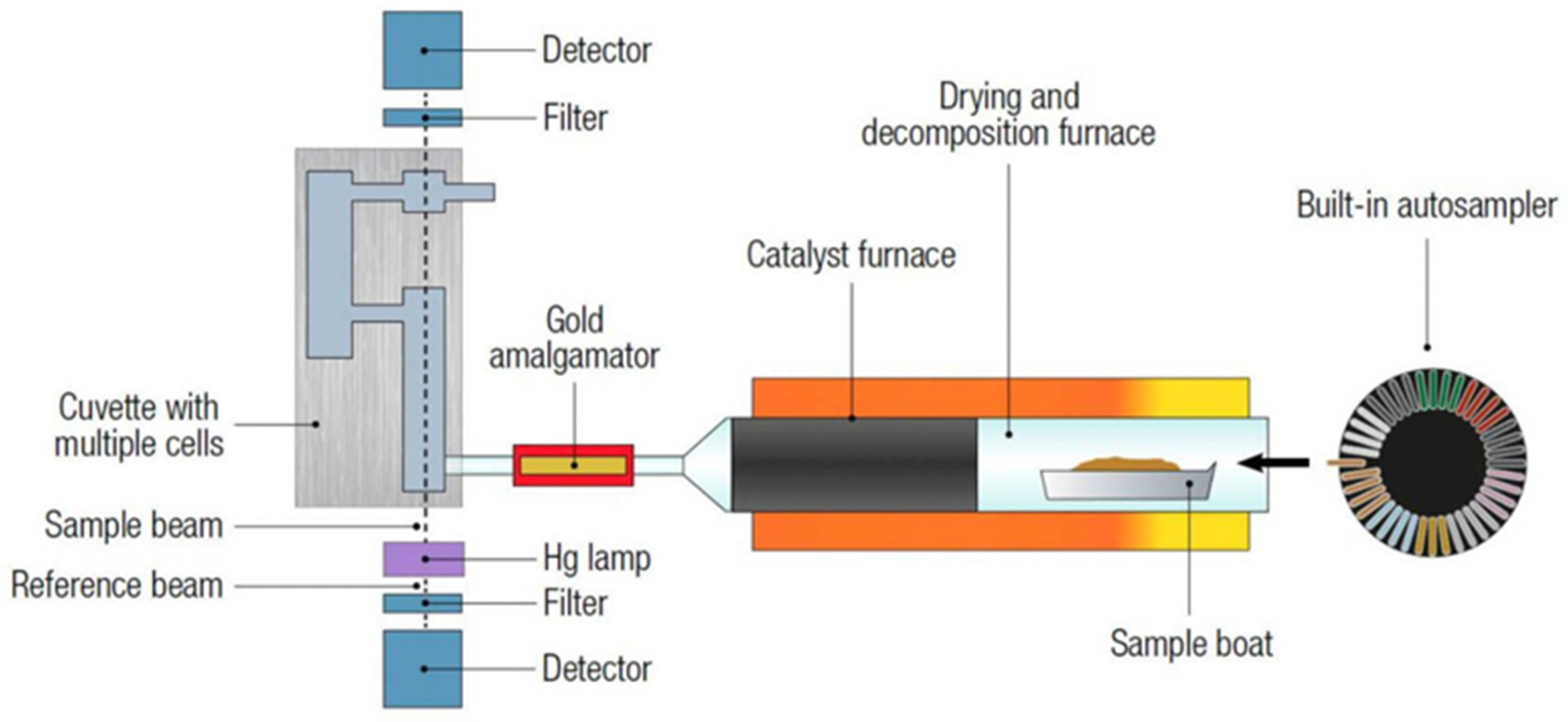
A direct mercury analyser (DMA) is an instrument designed to measure the concentration of mercury as shown in Figure 1, specifically in solid and liquid samples, without the need for complicated sample preparation. The technique offers a rapid and simple method for the determination of mercury in alternative solid fuels [6]. Taking into consideration that mercury is a toxic element, various methods have been developed to detect and quantify mercury in different samples. One of the classic and widely used methods for mercury determination is cold vapor atomic absorption spectrometry (CVAAS).
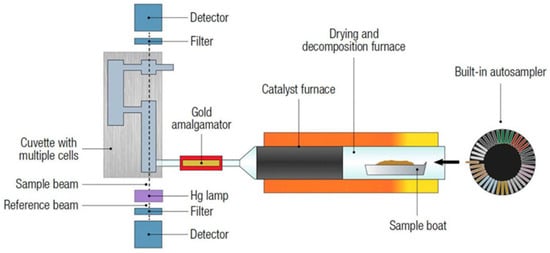
Figure 1.
A schematic of the DMA-80 evo direct mercury analyser [6].
This study aims to prove the potential upgrade of the lab by highlighting the benefits of using the direct mercury analyser instead of traditional methods for mercury determination.
2. Materials and Methods
2.1. Direct Mercury Analyser: A Technique Overview
The direct mercury analyser (DMA) is an instrument designed to measure the concentration of total mercury directly in solid, liquid, and even gas samples without the need for complicated sample preparation.
DMA requires minimal sample preparation since the samples can be introduced into the instrument directly and offer a rapid, within-minutes analysis. It reduces the use of toxic chemicals (such as the HF solution for digestion) and their potential waste and also reduces the risk of contamination due to the direct analysis and absence of wet chemistry [6].
In contrast, established techniques in elemental analyses, such as CVAAS and ICP, are more time-consuming due to the need for sample preparation (the digestion of solid samples with toxic and hazardous solutions). Furthermore, the multiple steps following this technique can introduce sources of final result errors, which may prove to be difficult to identify and eliminate. On the other hand, such techniques offer multi-element determination and have proved their accuracy [7].
2.2. Sample Preparation
The SRF samples were introduced in the lab, and they were dried in an oven at 105 °C for 24 h. After drying the samples, which were ground in a pulverised mill with five different sieves (8 mm, 2 mm, 1 mm, 0.5 mm, and 0.25 mm), each ground sample was weighed and moved into the direct mercury analyser.
2.3. Testing DMA
In order to determine the efficiency of the newly acquired DMA in our lab, two sets of tests were performed; one to identify sufficient accuracy and the other to specify the required particle size of solid samples. SRF samples were used in both sets, bearing in mind that their results are probably valid for other solid fuels that are more homogeneous, like solid biofuels and fossil fuels.
Two SRF reference materials (RMs) were measured; both RMs were samples of inter-laboratory test schemes. Their mean values were compared to the reference ones, taking into account the calculated uncertainty by the organiser of the schemes. Moreover, one liquid reference material was also measured using the same calibration and procedure.
The second set consisted of testing three SRF samples originating from ASR. Each sample was tested after being ground and sieved to 8 mm, 2 mm, 1 mm, 0.5 mm, and 0.25 mm. The 8 mm sub-sample is the raw sample in many cases. For each sample, the mercury concentration of the 0.25 mm particle size sub-sample is compared to the concentrations of the larger particle size sub-samples in order to determine up to what particle size one can accept the DMA results as statistically indifferent. Compared sub-samples are considered different materials with altered fuel properties. A hypothesis check is performed assuming that all samples are representative of populations (sources) where the mercury concentration follows a normal distribution while their variances are unknown and different. The null hypothesis is that the populations of the compared sub-samples have the same mean mercury concentration. The alternative is that they do not. Therefore, the quantity follows the t-distribution and is compared to the theoretical value of the two-sided t-distribution with 95% probability and for ν degrees of freedom [8]:
where m1 and m2 are the mean values of the compared sub-samples and sd is the typical error of the difference of the means:
where s1 and s2 are the standard deviations of the compared sub-samples and n1 and n2 are their measurements.
td = (m1 − m2)/sd
sd = √ (s12/n1 + s22/n2)
The degrees of freedom, ν, required for the theoretical value of the t-distribution is calculated as follows:
where u is defined as
ν = 1/(u2/(n1 − 1) + (1 − u)2/(n2 − 1))
u = (s12/n1)/(s12/n1 + s22/n2), for s12 > s22
3. Results and Discussion
The measurements of the SRF reference materials and the liquid RM are presented in Table 1, followed by their reference values and respective uncertainties. The specific names of the RMs are as follows: an SRF analytic proficiency test 2022 organised by Delta Coal Control GmbH (reference concentration: 0.1675 mg/kg), an SRF Schema 5121 proficiency test 2021 organised by Greek General Chemical State Laboratory (reference concentration: 0.382 mg/kg) and a wastewater Schema 2412 proficiency test 2023 organised also by the Greek General Chemical State Laboratory (reference concentration 0.137 mg/L). The DMA presents acceptable accuracy since the difference between the measured means and the reference values is smaller than the reported uncertainties.

Table 1.
Mercury concentrations of the reference material.
The measurements of the SRF samples that originated from ASR are presented in Table 2, Table 3 and Table 4. In each table, the values of five sub-samples that differed in particle size are presented, including their mean values and standard deviations, as well as the results of the above-defined quantities, td, sd, and ν (utilising Equations (1)–(4)). Each sub-sample is compared to one with a nominal particle size of 0.25 mm. The theoretical value of the t-distribution, tth, may be acquired from statistical tables. Comparing the absolute value of td with the two-sided t-distribution value, we concluded with a 95% probability that we must accept the null hypothesis for the 0.5 mm and 1 mm sub-samples, meaning that grinding down to 1 mm seems sufficient for most SRF cases, while grinding down to 2 mm might be enough for a few cases, as illustrated in Table 2 (when sample heterogeneity can be regarded as low).

Table 2.
Mercury concentrations of SRF sample 1 originating from ASR.

Table 3.
Mercury concentrations of SRF sample 2 originating from ASR.

Table 4.
Mercury concentrations of SRF sample 3 originating from ASR.
The direct mercury analyser (DMA) is an instrument that measures the concentration of total mercury in solid and liquid samples. The total mercury is trapped by an amalgamator as elemental mercury and subsequently measured using atomic absorption spectrometry; thus, measurements may consider the matrix as independent, and instrument calibration is sufficient for different sample materials.
4. Conclusions
As presented in this work, DMA requires minimal sample preparation compared to established techniques in elemental analyses while offering acceptable accuracy in the case of solid fuels. The analysis is complete within minutes, and it does not require the use of toxic chemicals, eliminating potential waste and reducing the analysis cost. The DMA can be regarded as an efficient laboratory tool, offering a robust alternative to atomic absorption spectrometry and Induced Coupling Plasma Spectrometry procedures, especially in terms of accuracy, speed, safety, and cost-effectiveness.
Author Contributions
P.S.: methodology, validation, investigation, writing—review and editing; A.T.: writing—review and editing; P.A.: writing—review and editing, project administration; I.K.: writing—review and editing; C.K.: writing—review and editing; P.G.: writing—review and editing, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
Partnership and Cooperation Agreement (NSRF) 2014-2020, European Regional Development Fund (ERDF) “Development of New Innovative Energy Technologies of Low Carbon Footprint for the Enhancement of Excellence in the Region of Western Macedonia”, MIS code 5047197 of The Operational Program “Competitiveness, Entrepreneurship & Innovation” (EPAnEK) co-financed by Greece and the European Union.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are available in a publicly accessible repository that does not issue DOIs. Publicly available datasets were analysed in this study. This data can be found here: https://excelwmac.gr/en (assessed on 29 August 2023).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Arina, D.; Bendere, R.; Kalnacs, J. Waste Products—RDF or SRF as Energy Source in EU. 2018. Available online: http://uest.ntua.gr/naxos2018/proceedings/pdf/NAXOS2018_Arina_etal.pdf (accessed on 1 October 2023).
- WHO. Mercury and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/mercury-and-health (accessed on 4 September 2023).
- Eur-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32020L2184&qid=1693832014111 (accessed on 4 September 2023).
- Ricel, K.M.; Walker, E.M., Jr.; Wu, M.; Gillette, C.; Blough, E.R. Environmental Mercury and Its Toxic Effects. J. Prev. Med. Public Health 2014, 47, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Zevenhoven, R.; Saeed, L. Automotive Shredder Residue (ASR) and Compact Disc (CD) Waste: Options for Recovery of Materials and Energy; Helsinki University of Technology, Department of Mechanical Engineering: Espoo, Finland, 2003. [Google Scholar]
- DMA-80 Evo: Operator Manual (MA 213). 24 September 2021. Available online: https://milestoneconnect.com/wp-content/uploads/connect/dma80/documents/Documentation/usermanual/DMA-80%20evo%20User%20Manual%20-%20MA213-003.pdf (accessed on 1 October 2023).
- SHIMADZU Atomic Absorption Spectrophotometer: AA-6300 Instruction Manual, (Hydride Vapor Generator Instruction Manual). Unpublished work. 2007.
- Navidi, W. Statistics for Engineers and Scientists, 3rd ed.; McGraw-Hill: New York, NY, USA, 2010. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).