The Synthesis, Characterization, and Antimicrobial Activity of Magnetite (Fe3O4) Nanoparticles by the Sol–Gel Method †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Magnetite Nanoparticles
2.3. Characterization
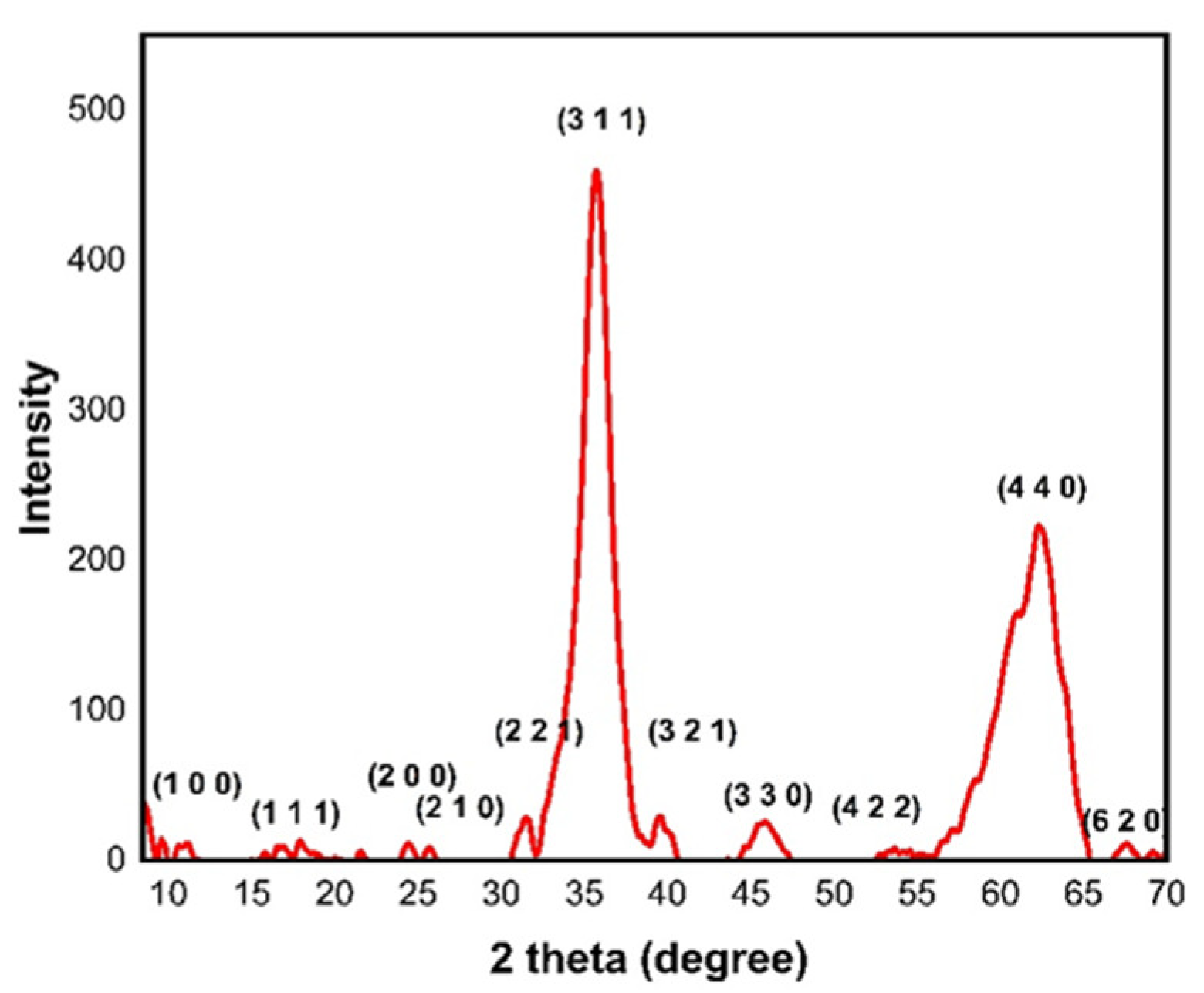
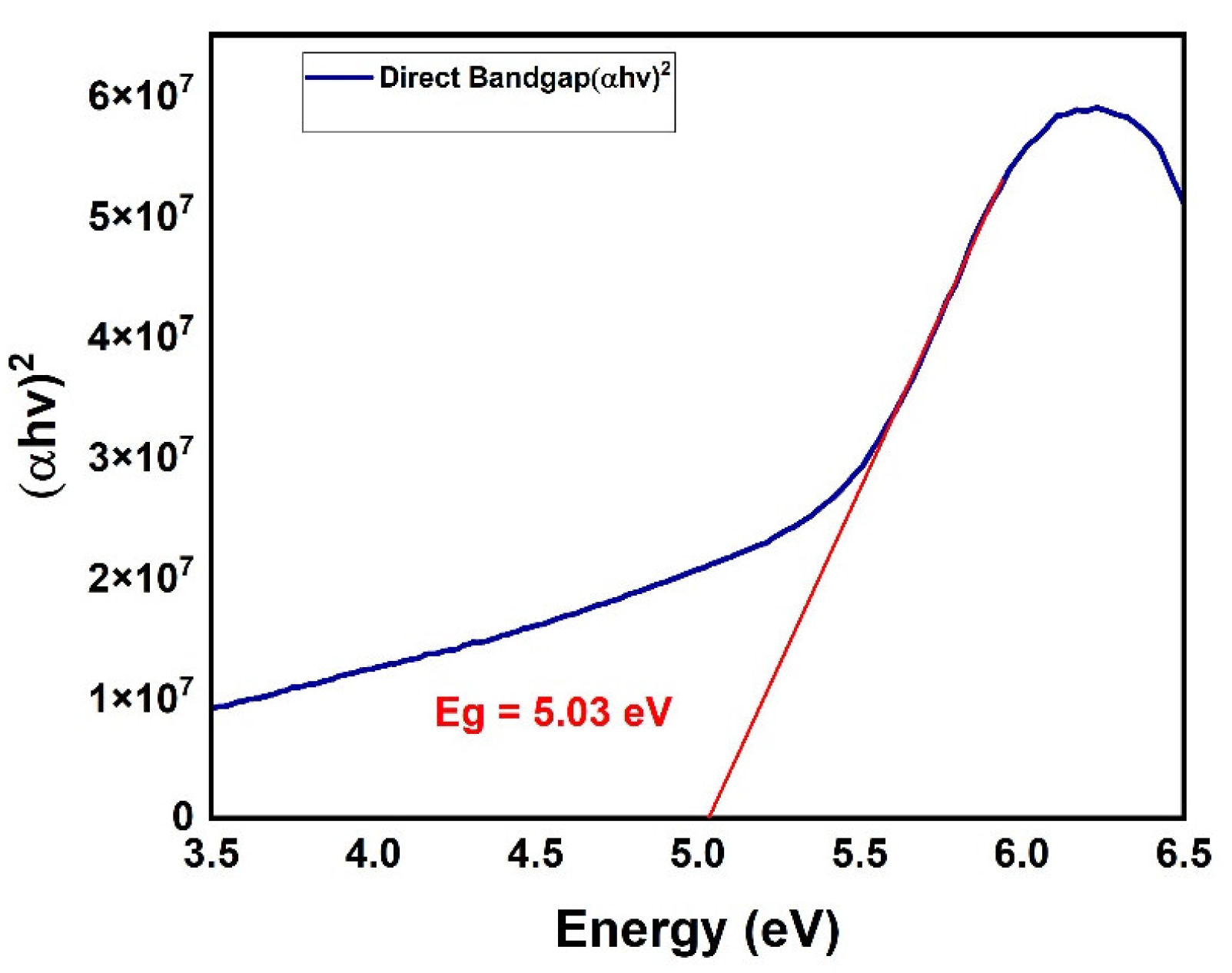
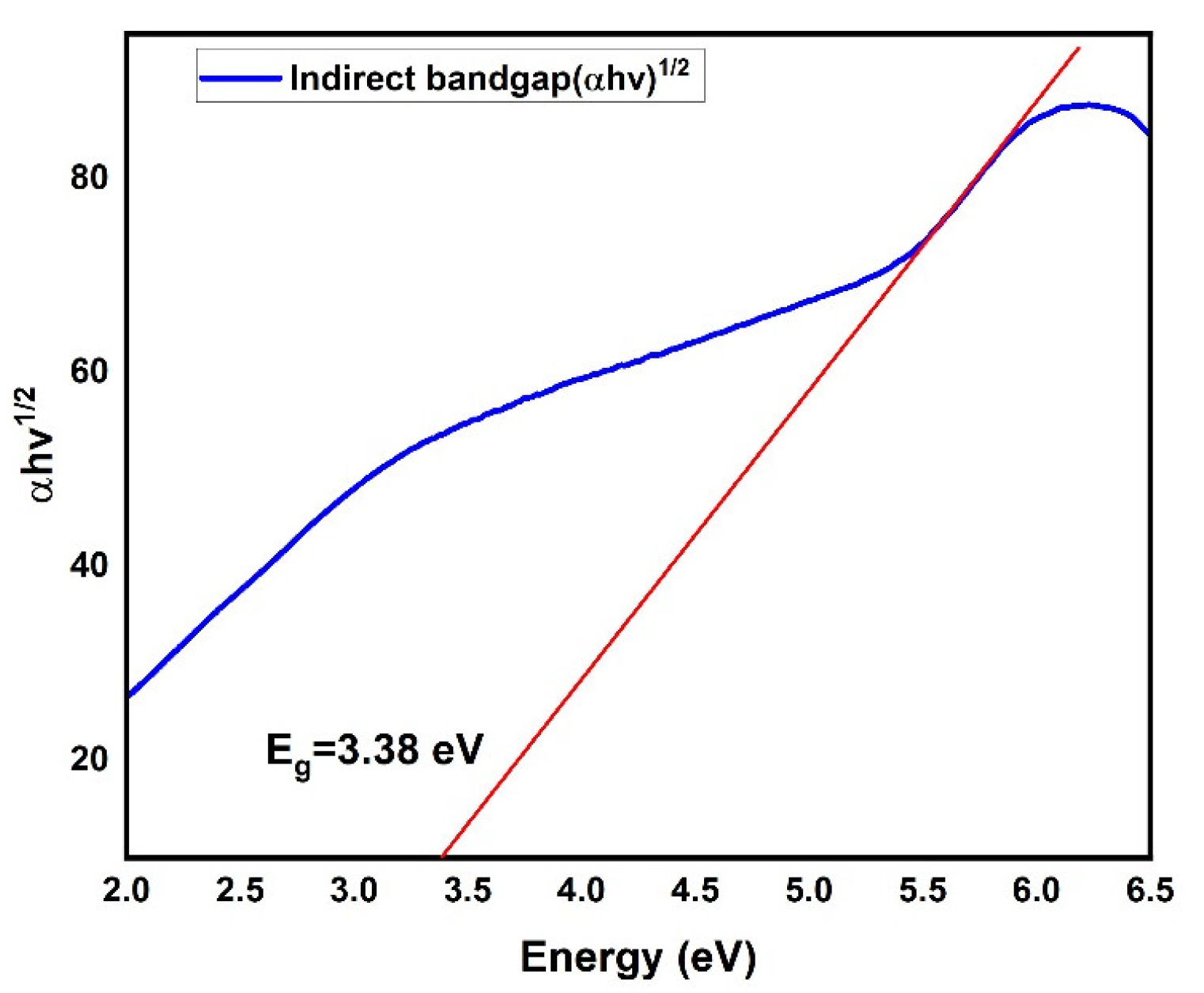
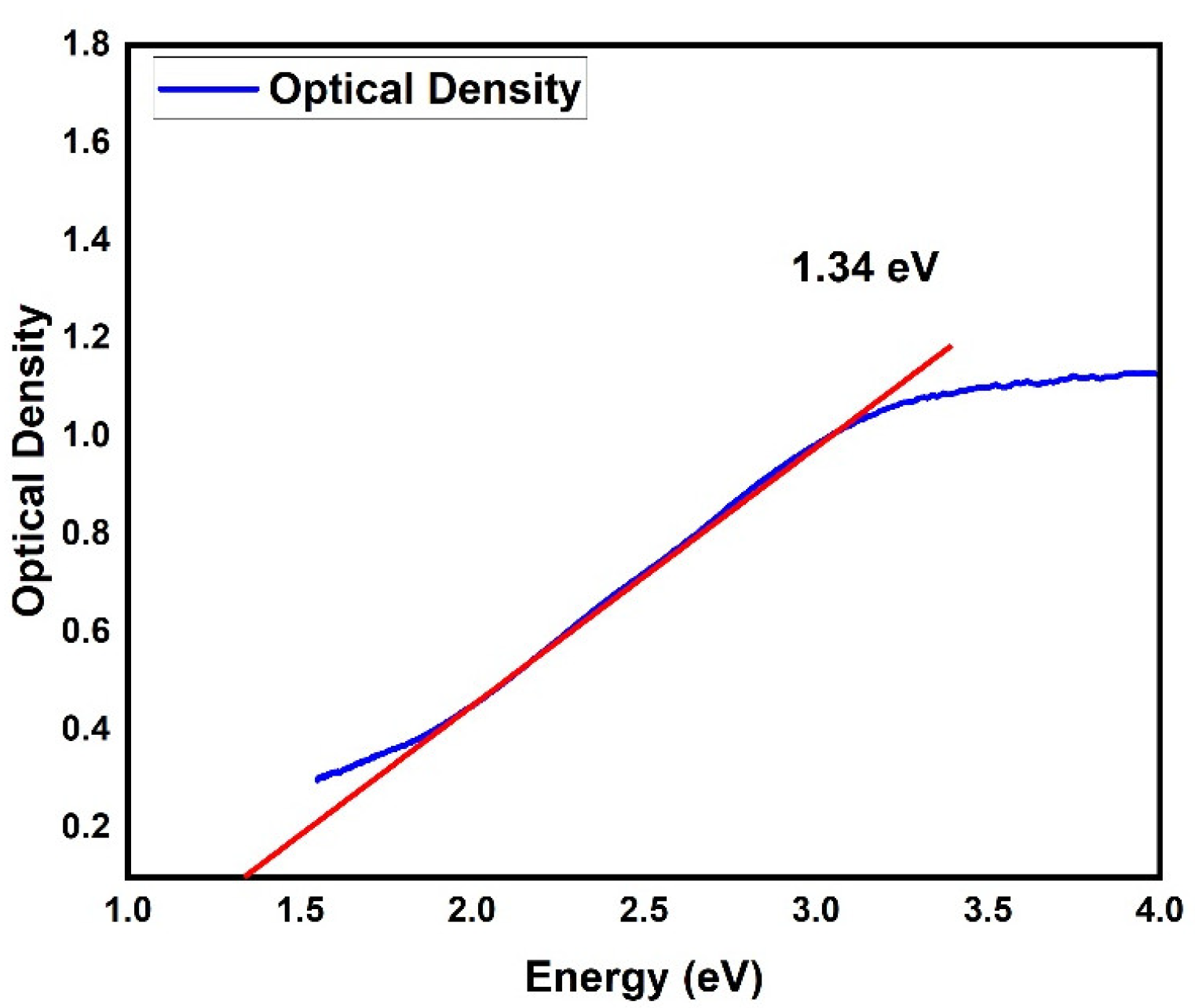
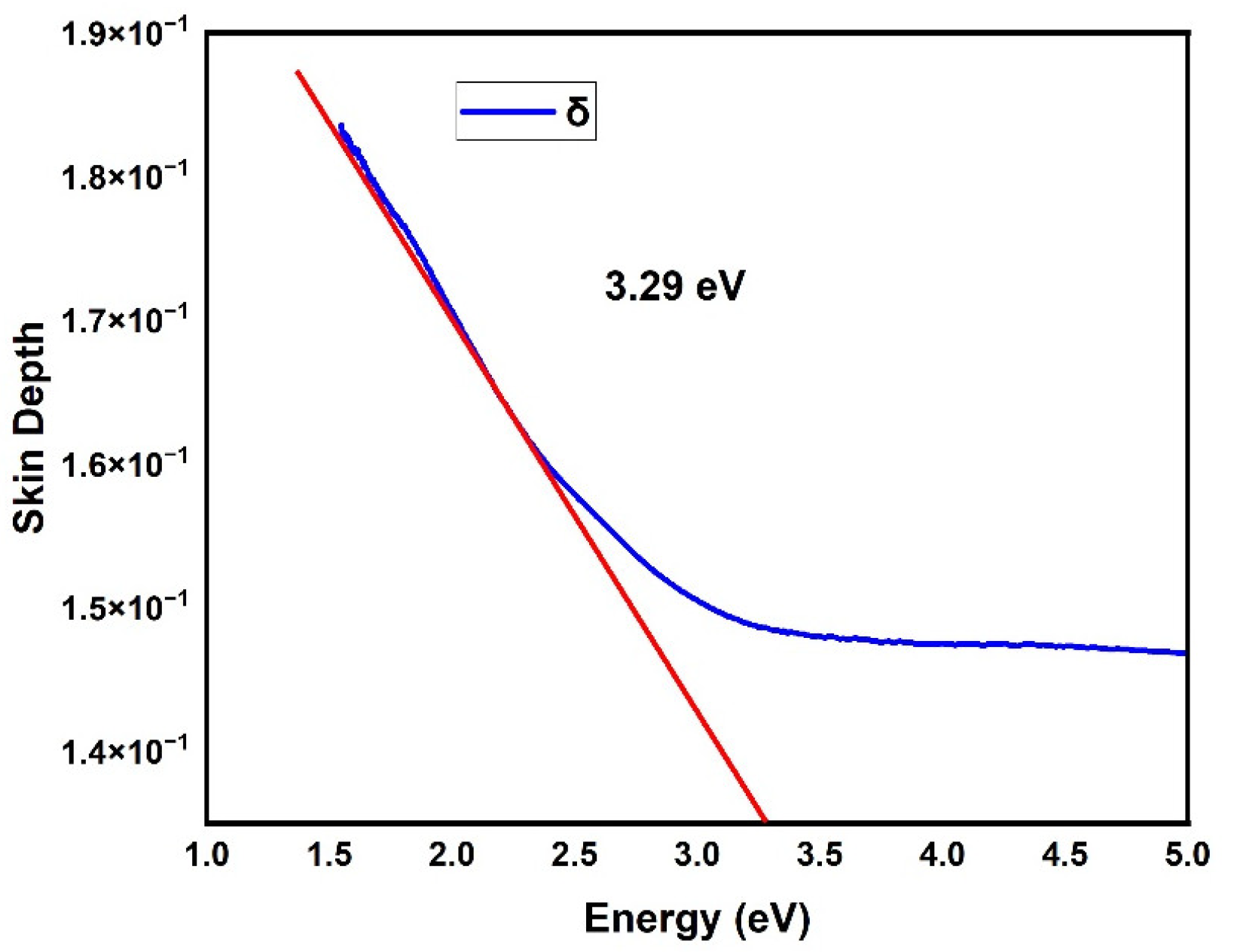
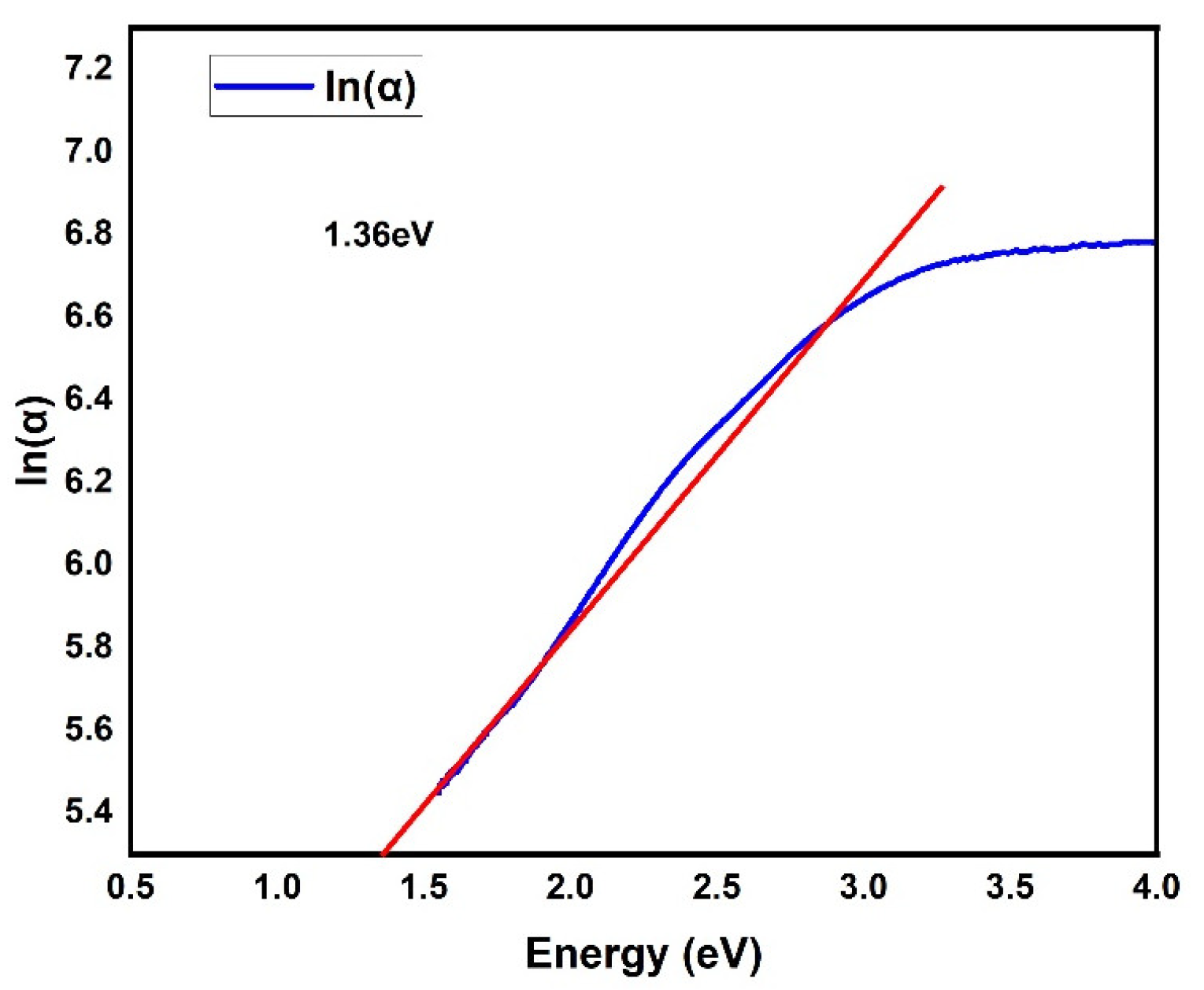
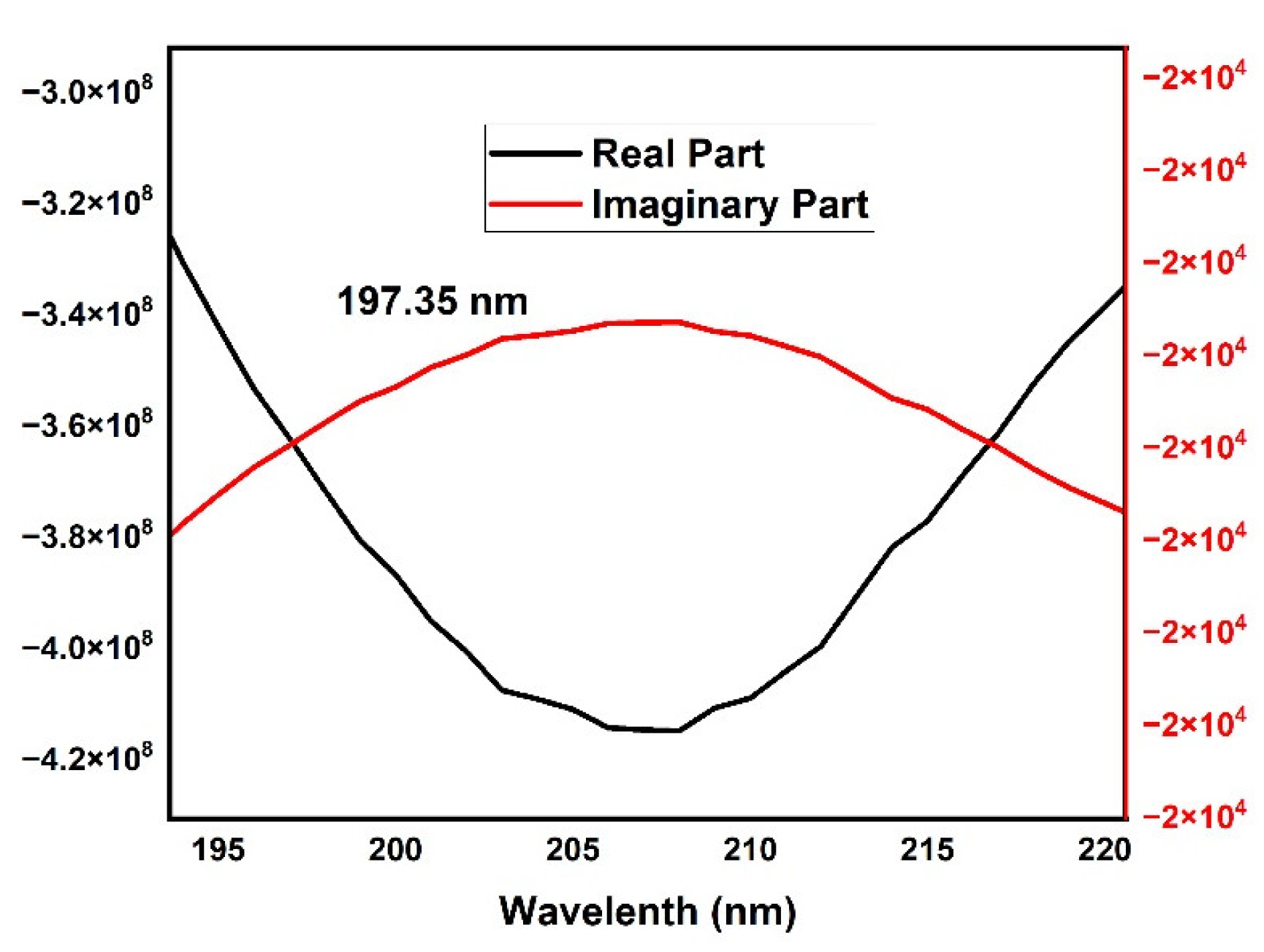
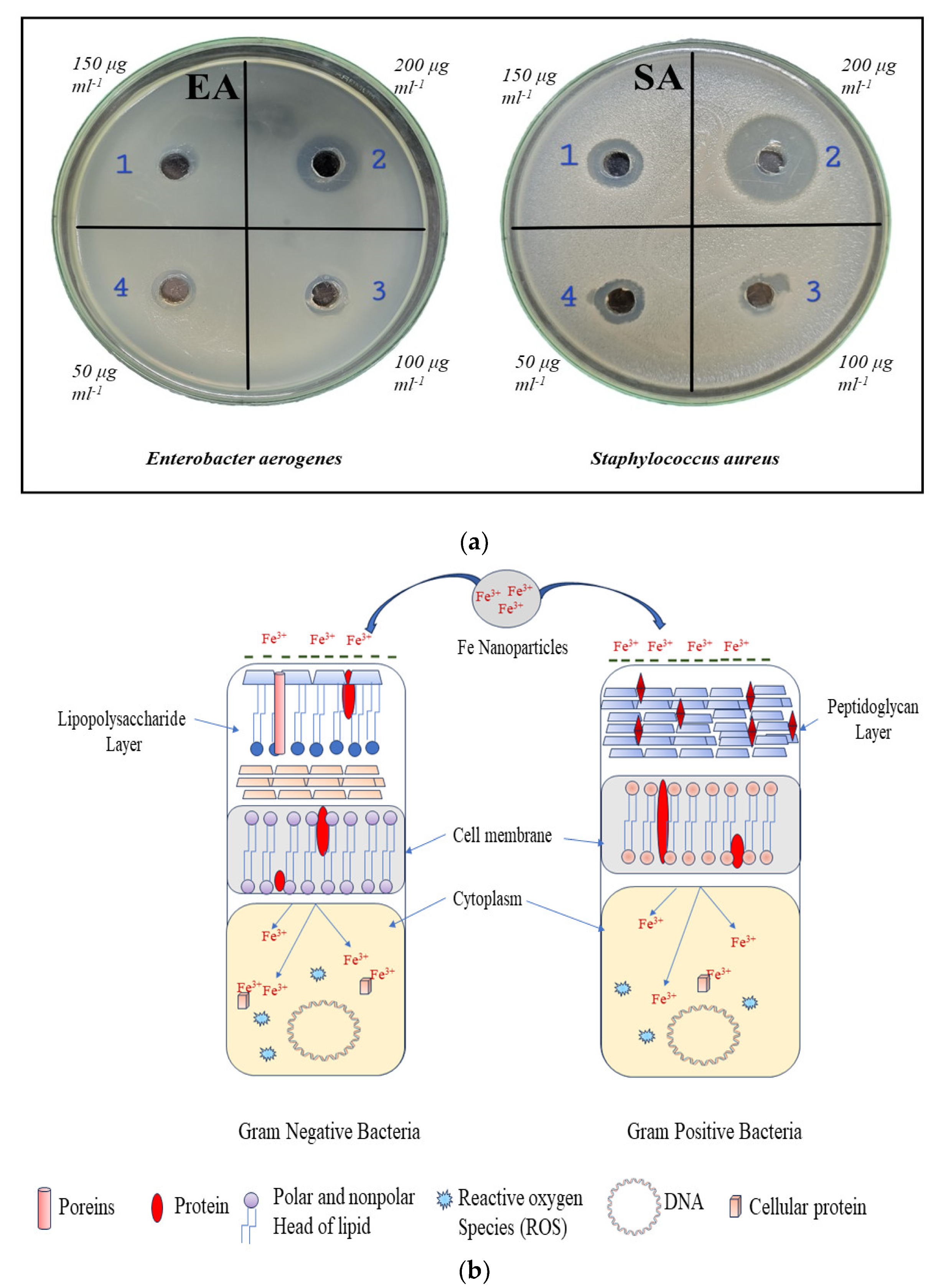
3. Results and Discussion
- Antimicrobial activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shah, S.T.; Chowdhury, Z.Z.; Johan, M.R.B.; Badruddin, I.A.; Khaleed, H.M.T.; Kamangar, S.; Alrobei, H. Surface Functionalization of Magnetite Nanoparticles with Multipotent Antioxidant as Potential Magnetic Nanoantioxidants and Antimicrobial Agents. Molecules 2022, 27, 789. [Google Scholar] [CrossRef] [PubMed]
- Lemine, O.; Omri, K.; Zhang, B.; El Mir, L.; Sajieddine, M.; Alyamani, A.; Bououdina, M. Sol–gel synthesis of 8nm magnetite (Fe3O4) nanoparticles and their magnetic properties. Superlattices Microstruct. 2012, 52, 793–799. [Google Scholar] [CrossRef]
- Cui, H.; Liu, Y.; Ren, W. Structure switch between α-Fe2O3, γ-Fe2O3 and Fe3O4 during the large scale and low temperature sol–gel synthesis of nearly monodispersed iron oxide nanoparticles. Adv. Powder Technol. 2013, 24, 93–97. [Google Scholar] [CrossRef]
- Hu, P.; Chang, T.; Chen, W.-J.; Deng, J.; Li, S.-L.; Zuo, Y.-G.; Kang, L.; Yang, F.; Hostetter, M.; Volinsky, A.A. Temperature effects on magnetic properties of Fe3O4 nanoparticles synthesized by the sol-gel explosion-assisted method. J. Alloy. Compd. 2018, 773, 605–611. [Google Scholar] [CrossRef]
- Wang, X.L.; Wei, L.; Tao, G.H.; Huang, M.Q. Synthesis and characterization of magnetic and luminescent Fe3O4/CdTe nanocomposites using aspartic acid as linker. Chin. Chem. Lett. 2011, 22, 233–236. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, R.; Liu, T.; Lv, H.; Zhou, L.; Zhang, X. One-pot synthesis of grass-like Fe3O4 nanostructures by a novel microemulsion-assisted solvothermal method. Ceram. Int. 2014, 40, 1059–1063. [Google Scholar] [CrossRef]
- Eom, Y.; Abbas, M.; Noh, H.; Kim, C. Morphology-controlled synthesis of highly crystalline Fe3O4 and CoFe2O4 nanoparticles using a facile thermal decomposition method. RSC Adv. 2016, 6, 15861–15867. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, G. One-step hydrothermal synthesis of magnetic Fe3O4 nanoparticles immobilized on polyamide fabric. Appl. Surf. Sci. 2012, 258, 4952–4959. [Google Scholar] [CrossRef]
- Bhaumik, M.; Maity, A.; Gupta, V.K. Synthesis and characterization of Fe0/TiO2 nano-composites for ultrasound assisted enhanced catalytic degradation of reactive black 5 in aqueous solutions. J. Colloid Interface Sci. 2017, 506, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Shaker, S.; Zafarian, S.; Chakra, C.S.; Rao, K.V. Preparation and characterization of magnetite nanoparticles by Sol-Gel method for water treatment. Int. J. Innov. Res. Sci. Eng. Technol. 2013, 7, 2969–2973. [Google Scholar]
- Hassanien, A.; Akl, A.A. Effect of Se addition on optical and electrical properties of chalcogenide CdSSe thin films. Superlattices Microstruct. 2016, 89, 153–169. [Google Scholar] [CrossRef]
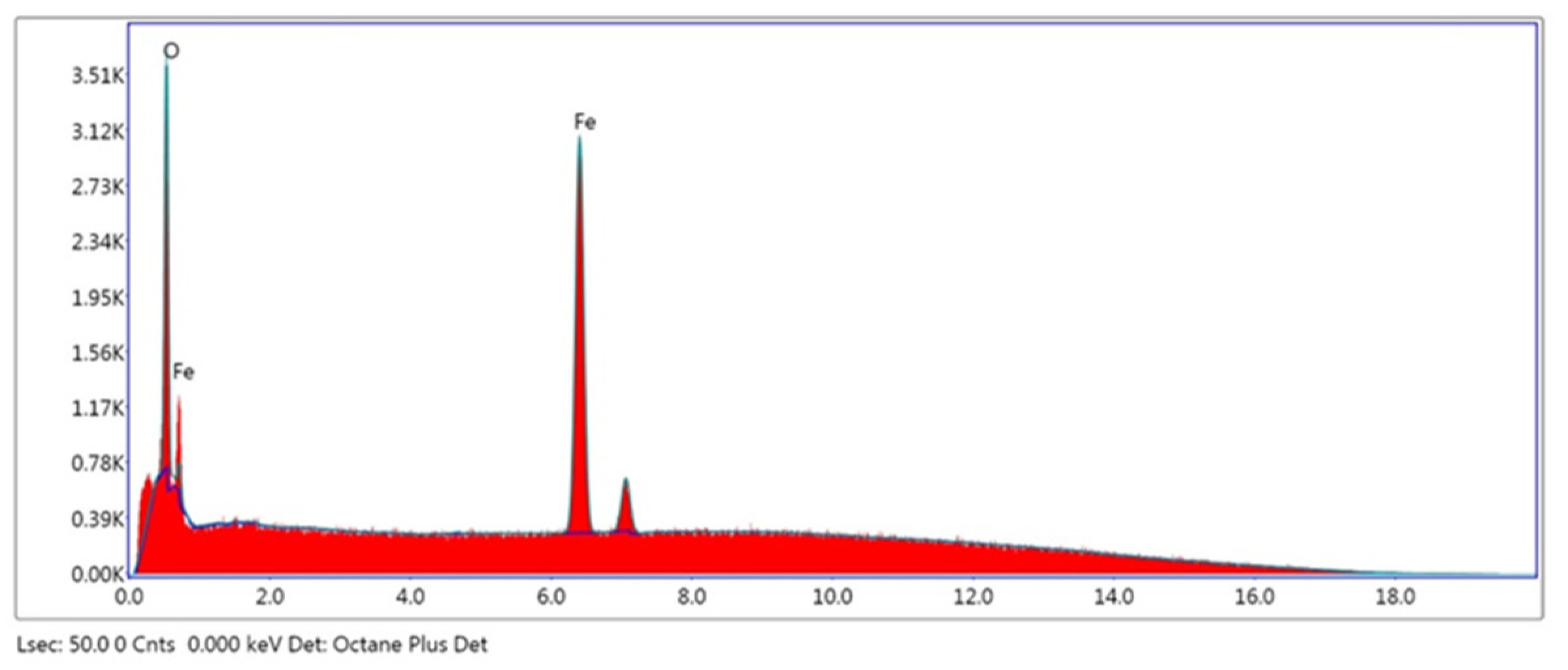
| Element | Weight% |
|---|---|
| O K | 24.7 |
| Fe K | 75.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brahmbhatt, P.N.; Bharucha, S.R.; Bhatt, A.; Dave, M.S. The Synthesis, Characterization, and Antimicrobial Activity of Magnetite (Fe3O4) Nanoparticles by the Sol–Gel Method. Eng. Proc. 2023, 56, 293. https://doi.org/10.3390/ASEC2023-15950
Brahmbhatt PN, Bharucha SR, Bhatt A, Dave MS. The Synthesis, Characterization, and Antimicrobial Activity of Magnetite (Fe3O4) Nanoparticles by the Sol–Gel Method. Engineering Proceedings. 2023; 56(1):293. https://doi.org/10.3390/ASEC2023-15950
Chicago/Turabian StyleBrahmbhatt, Priyansh N., Shivani R. Bharucha, Ashish Bhatt, and Mehul S. Dave. 2023. "The Synthesis, Characterization, and Antimicrobial Activity of Magnetite (Fe3O4) Nanoparticles by the Sol–Gel Method" Engineering Proceedings 56, no. 1: 293. https://doi.org/10.3390/ASEC2023-15950






