Abstract
Nanogel systems loaded with nanosuspension area promising approach tonose-to-brain delivery in order to reduce the dose and dosing frequency and also improve the bioavailability of the drug. In the present study, an attempt was made to develop a nanosuspension-loaded insitu nasal nanogel of nortriptyline HCl (NTH) to achieve effective administration through the intranasal route to reach the brain via the olfactory and trigeminal nerves to improve the therapeutic efficiency. A nanoprecipitation–ultrasonication method followed by high-pressure homogenization was elected for the preparation of the nanosuspension, which was further incorporated into the in situ gelling polymer solution. The optimized nanosuspension-loaded nanogels were prepared using gellan gum. The optimized formulation showed an average particle size of 10–100 nm, a good PDI value, an increase in solubility, a good gelation property, and the desired viscosity to adhere to the nasal mucosa after ionic interactions. In vitro drug release was found to be greater than a drug solution over a period of 60 min. Spreadability and viscosity studies showed better results in achieving a good residence time. Hence, it was proved that insitu nanogel is one of the best possible approaches for the targeting of drugs toward the brain in nanoform.
1. Introduction
One of the most well-known fields of nanotechnology studies is nanomedicine [1]. It helps in the development of specialized pharmaceutical treatments for disease diagnosis, prevention, and treatment using nanotechnology [2]. The size of a nanoparticle ranges from 10–1000 nm. The active pharmaceutical ingredient (API) is entrapped, encapsulated, dissolved, or linked to the nanoparticle matrix [3]. Although nanoparticulate drug delivery systems have several uses, some recent studies have concentrated on the delivery of drugs and treatments across the blood–brain barrier (BBB) [4]. There are several kinds of nanomedicine; however, nanosuspension has a primary emphasis on providing opportunities for drugs that are poorly soluble. Gellan gum (GG) is produced commercially via microbial fermentation using the microorganism Sphingomonas elodea or Pseudomonas elodea [5]. Even at low concentrations, GG has the ability to form a firm gel and exhibits bioadhesive properties. Gellan gum is capable of forming gels with monovalent (H+, K+, Na+) as well as divalent ions (Ca2+, Mg2+) [6].
The effectiveness of antidepressant therapy is based on drug concentrations and bioactivity; however, standard oral and parenteral medicines have limitations due to challenges in crossing the BBB [7]. Intranasal administration has the potential to bypass the BBB. This is due to the fact that the trigeminal nerves and olfactory region of the brain are directly connected to the nasal cavity [8,9].
Nortriptyline hydrochloride (NTH), a tricyclic antidepressant, is indicated in the treatment of major depression. Some of the NTH-related challenges, such as its poor oral bioavailability (around 50%) and fluctuating plasma levels, could prevent patients from complying with treatment and lead to failure of therapy.
The purpose of this research is to prepare and optimize an intranasal formulation by incorporating a nortriptyline HCl nanosuspension into insitu gel for nose-to-brain delivery. The research focuses on the development of nasal gels loaded with poorly water-soluble drug formulations for intranasal delivery.
2. Materials and Methods
The nortriptyline HCl (NTH) was provided as a gift sample by Micro Labs Pvt. Ltd., Mumbai, India. The excipients that were used in the research, such as the tween 80, poloxamers, HPMC K4M, gellan gum, and paraben, and solvents such as methanol, ethanol, and DMSO (analytical-grade) were purchased from ResearchLab Fine Chem. Industries, Mumbai. The water used for the experiments was distilled.
2.1. FTIR Analysis
FTIR studies were performed using a FTIR instrument (IR Affinity-1, Shimadzu, Kyoto, Japan). In this, the sample was placed on the area of detection, and the FTIR spectrum and peak table were recorded.
2.2. Preparation of Nanosuspension Loaded with Nortriptyline HCl
The nanosuspension was prepared using the nanoprecipitation—ultrasonication method followed by a high-pressure homogenizer (PandaPLUS2000 homogenizer, GEA, Niro Soavi, Italy). During nanoprecipitation–ultrasonication, the organic phase, consisting of NTH and varying concentrations of different surfactants (tween 80, poloxamer 407, and poloxamer 188) in methanol, was incorporated into the aqueous phase, i.e., 20 mL of distilled water containing stabilizer HPMC K4M with 10 min of sonication using a probe sonicator (Model no.ATP250, Athena Technology, Thane, India). The prepared nanosuspension was further passed through a homogenizer to achieve the desired particle size using optimization at 800 bar, suitable for nose-to-brain delivery (Table 1) [10,11].

Table 1.
Formulation table of nortriptyline HCl nanosuspension.
2.3. Characterization of Nanosuspension Loaded with Nortriptyline HCl
2.3.1. Entrapment Efficiency
The nortriptyline-HCl-loaded nanoparticles were separated from the aqueous suspension using centrifugation in a cooling centrifuge (Remi C24 Plus, International Scientific Instrument Co., Delhi, India) at 10,000 rpm and 4 °C for 20 min. The supernatant was collected, and the drug content (free drug) in the supernatant and with suitable dilutions and absorbance was determined using the UV spectrophotometer (Shimadzu-1800, Shimadzu, Kyoto, Japan) at 240 nm and calculated [12].
2.3.2. Solubility Studies
The saturation solubility was measured by adding an excess amount of the drug to each vial of a specific solvent and sealing it with a stopper. These vials were attached to an orbital shaker (Accumax (AI-123), Accumax India, Delhi, India) for 24 h at a speed of 50 rpm, and a temperature of around 37 ± 5 °C was maintained throughout the process. Each solution from the vials with suitable dilutions was scanned in a UV-visible spectrophotometer at 240 nm and its solubility calculated [11,12].
2.3.3. Particle Size, Polydispersibility Index (PDI), and Zeta Potential
The particle size distribution during process optimization was measured using a Mastersizer 2000 laser diffractometer (Malvern ZS 3000, Malvern Instrument Ltd., Worcestershire, UK). The zeta potential of the optimized batches was determined using a Zetasizer (Malvern ZS 3000, Malvern Instrument Ltd., Worcestershire, UK). The nanosuspension samples for analysis were prepared by diluting them appropriately with distilled water [11,12].
2.3.4. TEM Analysis
The particle size and the optimization of the nanosuspensions on the basis of the morphology of the nanoparticles were determined using transmission electron microscopy (TEM). The nanosuspensions were placed on a carbon-coated grid that was placed on a paraffin sheet, and the samples were left to allow the nanoparticles to adhere to the carbon substrate. Then, the grid was stained using phosphotungstate for 20 s. The prepared samples were dried under an IR lamp and then examined using TEM (Tecnai G2 Spirit BioTWIN, FEI Company, Eindhoven, The Netherlands). The photographic images were captured at different magnifications using the Soft Imaging Viewer software (Gatan Microscopy Suite-GMS3) [12].
2.4. Preparation of Nanosuspension-Loaded In Situ Nasal Nanogel
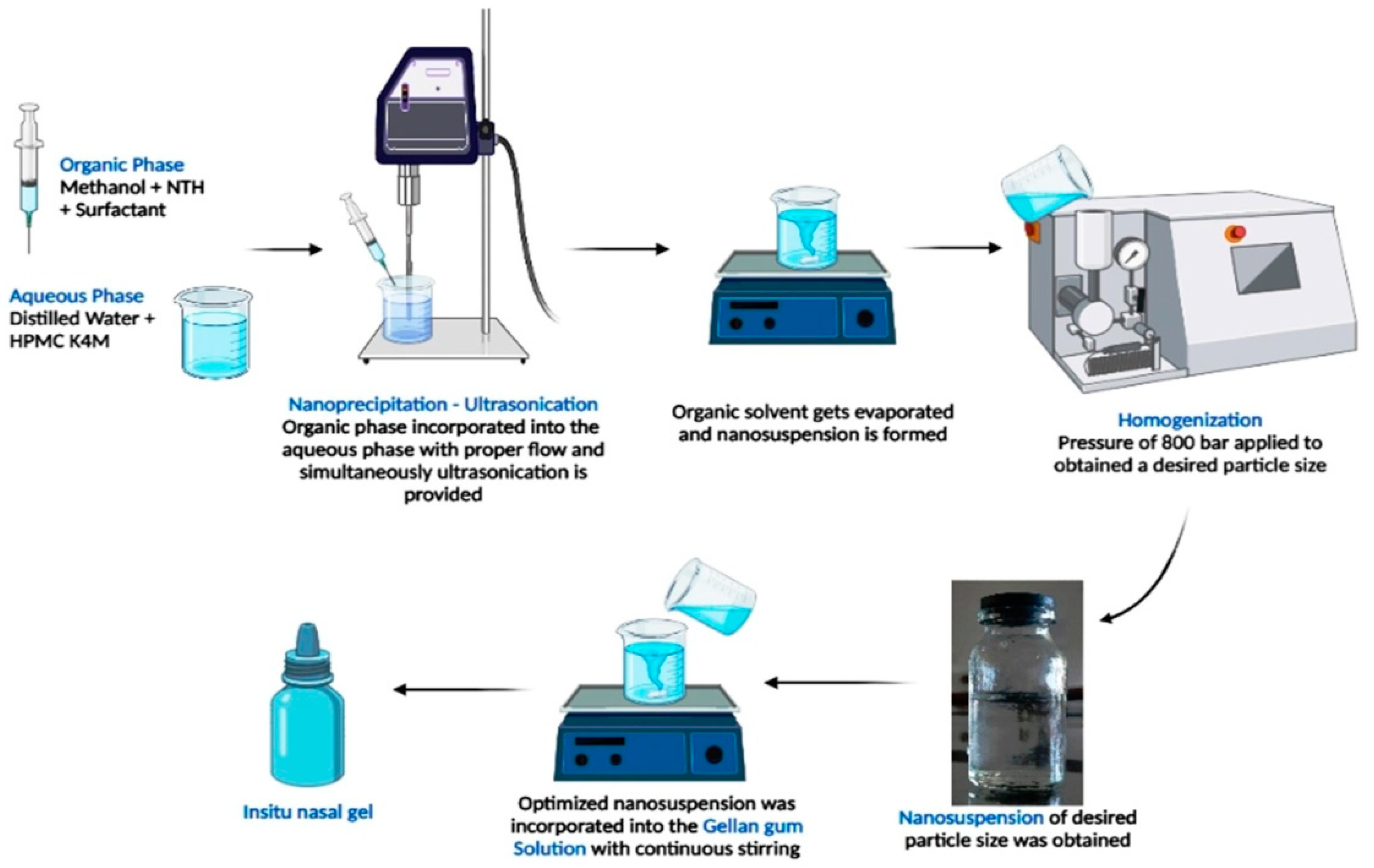
The deacetylated gellan gum solution was prepared using different concentrations of gellan gum (500, 1000, and 1500 mg) that were completely dissolved in distilled water using a magnetic stirrer at 300 rpm. On the other hand, PEG 400 (upto 4 mL) was added to the optimized nanosuspension (calculated volume) to obtain a clear sol-gel and to minimize the interactions of ions with the gellan gum solution before administration into the nasal cavity. Both solutions were mixed with the addition of methyl paraben (10 mg) as a preservative (Table 2). Finally, the formulation was stored in a properly capped glass container at room temperature and evaluated (Figure 1) [13].

Table 2.
Formulation table of insitu nasal gel.
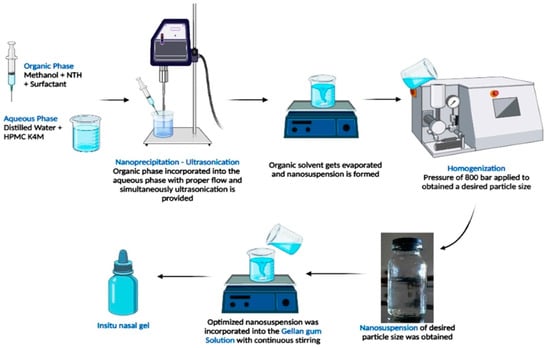
Figure 1.
Schematic representation of preparation of NTH-nanosuspension-loaded insitu nasal gel.
2.5. Characterization of Nanosuspension-Loaded In Situ Nasal Nanogel
2.5.1. Clarity
The clarity of the formulated insitu gels was determined via visual inspection under a black and white background, and it was graded as follows: turbid, +; clear, ++; and very clear (glassy), +++.
2.5.2. Drug Content
The appropriate amount of the formulation was diluted in the appropriate solvent, and the drug content was determined by using a UV-visible spectrophotometer at λ max 240 nm [13].
2.5.3. pH
A digital pH meter (Hanna, Italy, probe type) was used for the determination of the pH.In a beaker, 20 mL of each formulation was taken, and a glass electrode was sufficiently dipped into the samples of formulations. Then, the pH of the solution was recorded [13].
2.5.4. Spreadability
Spreadability is the area travelled per unit time (cm2/min) by the sol-gel formulation. Whatman filter paper was used for the determination of the spreadability of solution formulations NSG1 to NSG3. A 1 mL graduated pipette with a rubber bulb was clamped vertically to the stand in such a way that the tip of the pipette was 2 cm above the horizontal surface of the round-shape filter paper. Then, 0.1 mL of the sol formulation was dropped onto the center of the filter paper. At a fixed time interval of 20 s, the surface area covered by the formulation was measured [13].
2.5.5. In Vitro Gelation Study
The in vitro gelling capacity was determined by placing a freshly prepared solution of the in situ gels in a vial containing freshly prepared simulated nasal fluid (pH 5.5–6.5) ina ratio of 1:1 and equilibrating it at 37 °C. A visual assessment of the gel formation was carried out. The time required for the gelation to form gel was noted [13].
2.5.6. In Vitro Release
In vitro studies of the prepared formulations were carried out using Franz diffusion cells. The diffusion membrane was placed between the donor and receiver compartments. The donor compartment contained the prepared formulations and the receiver compartment containing the simulated nasal fluid (SNF) was maintained at 37 ± 0.5 °C. At specific time intervals, 1 mL of the sample was withdrawn from the receptor compartment and replaced with fresh SNF to maintain the sink condition. The samples withdrawn were filtered, and the amount of drug permeated was determined using a UV-visible spectrophotometer at λ max 240 nm [13]. A comparison between the drug solution, nanosuspension, and optimized insitu gel was undertaken. Kinetic models were applied using DDSolver 1.0 (software) to study the drug release.
2.5.7. Viscosity
A Brookfield DV2T digital viscometer (DVT2 Model, Brookfield Engineering Laboratories, Middleboro, MA, USA), spindle no. 64, with the RheocalcT 1.2.19 software was used for the determination of the viscosity of the prepared formulation. Viscosity measurements were taken before (at a neutral pH) and after gelling (at SNF pH 5.5–6.5) [14].
3. Results and Discussion
3.1. FTIR Analysis
A preformulation study was performed using FTIR to determine the compatibility between the nortriptyline HCl and formulation excipients.
In the FTIR spectrum of the nortriptyline HCl, NH stretching was observed at 2932.45 cm−1. CC stretching was found at 1591.74 cm−1. CN stretching was found at 1055.45 and 1158.15 cm−1. The peaks attributed to CH stretching were observed at 753.08 cm−1 and 804.43 cm−1. The observed peaks confirm that the drug is in pure form and the observed peaks also match the standard reported peaks.
In the FTIR spectrum of the optimized formulation, the peaks found at 2939.52 cm−1 and 1589.34 cm−1 resemble the NH stretching and CC stretching, respectively. The peaks observed at 1056.99 cm−1 and 1157 cm−1 are attributed to CN stretching, while the peak at 748.12 cm−1 shows CH stretching. All of the above peaks resemble the confirmation of the drug in the optimized formulation. Therefore, FTIR studies illustrated that the drug does not interact with any of the excipients and has compatibility within the formulation.
3.2. Characterization of Nanosuspension Loaded with Nortriptyline HCl
3.2.1. Entrapment Efficiency
During the studies, it was found that the 1:2 (drug: polymer) ratio gives an increase in entrapment but not significant as compared to 1:1; also, reported studies state that an increase in the polymer concentration causes an increase in particle size. Therefore, the 1:1 ratio is found to be optimized and gives an entrapment efficiency of 93.24 ± 1.23% for P407 (NS8).
3.2.2. Solubility Studies
The solubility of the NTH was increased at a nasal pH by preparing the nanosuspension 4-fold for P407 (NS8).
3.2.3. Particle Size, Polydispersibility Index (PDI), and Zeta Potential
The average particle size of the optimized formulation (NS8) was found to be 96.6 ± 16.24 nm, and the PDI was found to be 0.252, which demonstrates that the prepared nanoparticles are able to penetrate for brain delivery and are also homogenous. The zeta potential of the optimized formulation (NS8) was found to be −23.8 ± 5.64 mV. The zeta potential study shows that the particles are stable in the optimized nanosuspension (NS8).
3.2.4. TEM Analysis
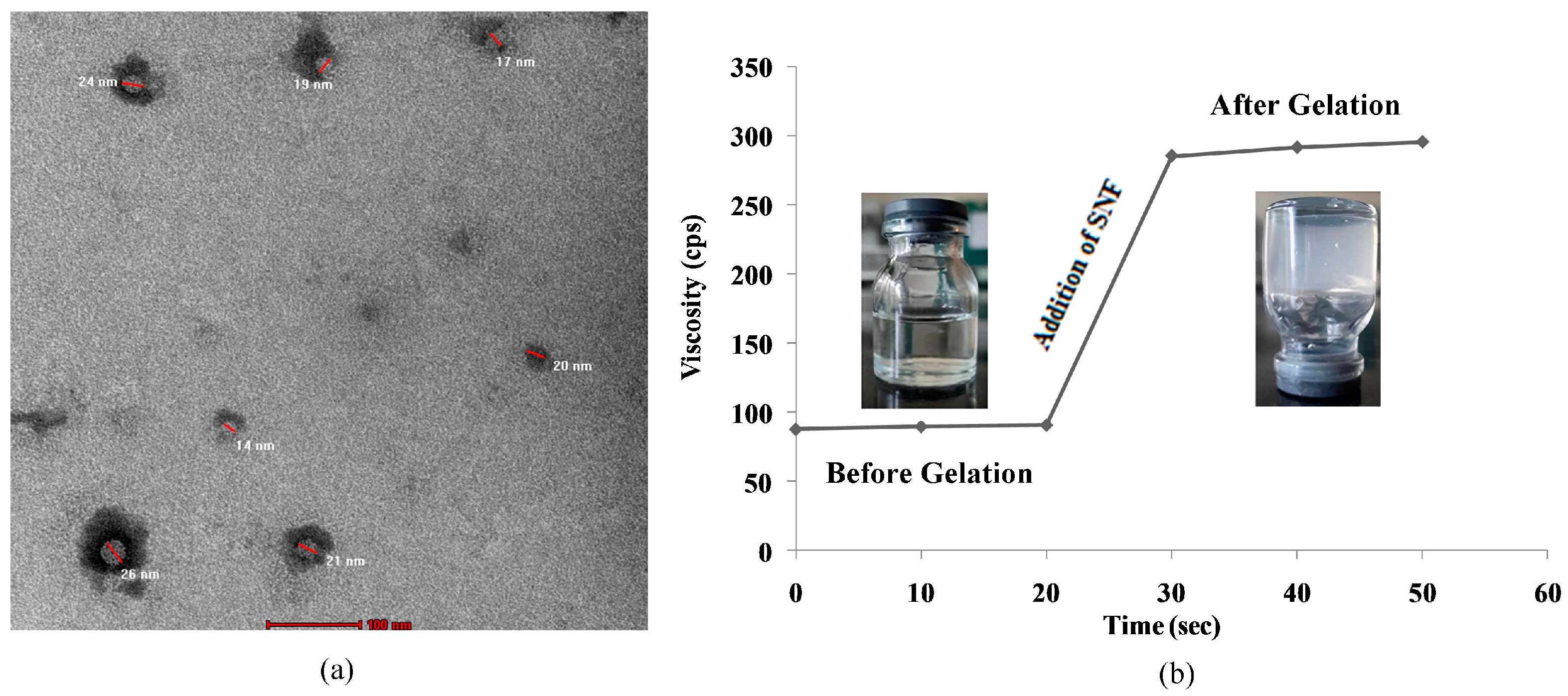
In the TEM image, it is clearly observed that the prepared nanoparticles are in the range of 10–100 nm (Figure 2a) in the optimized nanosuspension (NS8). Thus, the particle size studied using the zeta potential and TEM shows that the desired particle size is achieved.
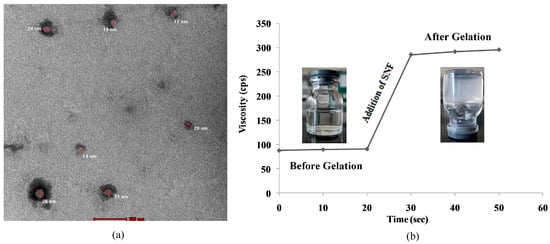
Figure 2.
(a) TEM microscopy of optimized nanosuspension; (b) gelation study at nasal pH of insitu nasal gel.
According to the performed studies, the poloxamer 407 batch exhibits the optimum results. Hence, NS8 is considered for the preparation of nanosuspension-loaded insitu nasal nanogel.
3.3. Characterization of Nanosuspension-Loaded In Situ Nasal Nanogel
3.3.1. Clarity
The clarity of the formulated insitu gels was determined using visual inspection and founds to be very clear for NSG1 as compared to NSG2 and NSG3 (Table 3).

Table 3.
Different parameter studies of prepared insitu gel.
3.3.2. Drug Content
The % drug content of the prepared insitu gels was found to be in the range of 97.14 ± 1.8% to 98.21 ± 1.5% (Table 3). NSG1 shows the highest percentage of drug content.
3.3.3. pH
The pH of all the prepared formulations was found to be 5.5 to 5.9 within the nasal pH range, which proved that the all insitu gels are compatible with a nasal pH to reduce irritation during administration (Table 3).
3.3.4. Spreadability
It is very important for insitu gels to have suitable spreadability to administer them easily and for them to spread easily on the nasal mucosa without leakage after administration. From the prepared insitu gels, NSG1 shows good spreadability as compared to NSG2 and NSG3 (Table 3).
3.3.5. In Vitro Gelation Study
Gelation studies were carried out using simulated nasal fluid with the formulation in a 1:1 ratio. All the prepared formulations show gelation within 50 s (Table 3) (Figure 2b). NSG1 consumes less time as compared to NSG2 and NSG3. Thus, this shows that insitu formed gel preserves its integrity without dissolving or eroding so as to localize the drug at the absorption site for an extended duration or to overcome mucociliary clearance.
From the above studies, it is revealed that NSG1 was found to be the superior formulation. Hence, NSG1 was taken into consideration for the in vitro release and viscosity study.
3.3.6. In Vitro Release
The release of the drug solution was found to be 49.04 ± 2.11% and the release of the nanosuspension was found to be more like 80.55 ± 2.14%. Furthermore, the final formulation shows a maximum release of 89.93 ± 2.08% in 60 min. This comparison of the drug release shows that the permeation of the drug increases with a decrease in the particle size of the drug by preparing a nanoform. The kinetic studies (with the DD-Solver 1.0 software) show that the formulation follows the Korsmeyer–Peppas model.
3.3.7. Viscosity
Viscosity increases with an increase in the concentration of the gelling agent, as was observed in the prepared formulations. To instill the formulation into the nasal cavity, the viscosity should be optimized and also undergo a rapid sol-gel transition due to ionic interaction. Hence, NSG1 fulfilled these properties of viscosity as compared to the others.
4. Conclusions
In the current research work, we explained how gellan gum, an anionic polymer that forms gel via ionic interactions, was used to develop a nortriptyline HCl nanosuspension-loaded insitu gel for nasal administration. This formulation demonstrates an effective drug release of about 89.93%, which might increase bioavailability and therapeutic effectiveness. The particle size was less than 100 nm, and the sol-gel transformation took less than 50 s, which exhibits superior outcomes for intranasal delivery. This suggests that a NTH-loaded nanosuspension-based insitu gel is able to deliver drug directly to the brain through the nose. The present article is expected to help scientists in developing an in situ gelling system for brain and lung delivery to improve the efficacy of treatments for brain-and lung-related disorders, as well as helping to enhance the bioavailability of drugs through the intranasal route.
Author Contributions
Conceptualization, B.R.R. and A.J.A.; methodology, B.R.R. and A.J.A.; software, B.R.R. and A.J.A.; formal analysis, B.R.R. and A.J.A.; investigation, B.R.R. and A.J.A.; resources, B.R.R. and A.S.J.; data curation, A.J.A. and B.R.R.; writing—original draft preparation, B.R.R. and A.J.A.; writing—review and editing, A.J.A., B.R.R. and A.S.J.; visualization, B.R.R. and A.S.J.; supervision, A.S.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
This work was encouraged by Shri D.D. Vispute College of Pharmacy and Research Center, Panvel, India.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Soares, S.; Sousa, J.; Pais, A.; Vitorino, C. Nanomedicine: Principles, properties, and regulatory issues. Front. Chem. 2018, 6, 360. [Google Scholar] [CrossRef] [PubMed]
- Sim, S.; Wong, N. Nanotechnology and its use in imaging and drug delivery (Review). Biomed. Rep. 2021, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.A.A.; Saleh, A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 2018, 26, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Ahlawat, J.; Guillama Barroso, G.; MasoudiAsil, S.; Alvarado, M.; Armendariz, I.; Bernal, J.; Carabaza, X.; Chavez, S.; Cruz, P.; Escalante, V.; et al. Nanocarriers as potential drug delivery candidates for over coming the blood–brain barrier: Challenges and possibilities. ACS Omega 2020, 5, 12583–12595. [Google Scholar] [CrossRef] [PubMed]
- Milivojevic, M.; Pajic-Lijakovic, I.; Bugarski, B.; Nayak, A.K.; Hasnain, M.S. Gellan gum in drug delivery applications. Nat. Polysacch. Drug Deliv. Biomed. Appl. 2019, 1, 145–186. [Google Scholar] [CrossRef]
- Rudko, M.; Urbaniak, T.; Musiał, W. Recent developments in ion-sensitive systems for pharmaceutical applications. Polymers 2021, 13, 1641. [Google Scholar] [CrossRef] [PubMed]
- Zorkina, Y.; Abramova, O.; Ushakova, V.; Morozova, A.; Zubkov, E.; Valikhov, M.; Melnikov, P.; Majouga, A.; Chekhonin, V. Nanocarrier drug delivery systems for the treatment of neuropsychiatric disorders: Advantages and Limitations. Molecules 2020, 25, 5294. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Minko, T. Nanotherapeutics for Nose-to-Brain Drug Delivery: An approach to by pass the blood brain barrier. Pharmaceutics 2021, 13, 2049. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Tao, J.; Wang, J. Design and application in delivery system of intranasal antidepressants. Front. Bioeng. Biotechnol. 2020, 8, 626882. [Google Scholar] [CrossRef] [PubMed]
- Saindane, N.S.; Pagar, K.P.; Vavia, P.R. Nanosuspension based in situ gelling nasal spray of carvedilol: Development, in vitro and in vivo characterization. AAPS PharmSciTech 2012, 14, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Sahu, B.P.; Das, M.K. Nanoprecipitation with sonication for enhancement of oral bioavailability of furosemide. Acta Pol. Pharm. 2014, 71, 129–137. [Google Scholar] [PubMed]
- Jadhav, P.; Yadav, A. Formulation, optimization, and in vitro evaluation of polymeric nanosuspension of flurbiprofen. Asian J. Pharm. Clin. Res. 2019, 12, 183–191. [Google Scholar] [CrossRef]
- Galgatte, U.C.; Kumbhar, A.B.; Chaudhari, P.D. Development of in situ gel for nasal delivery: Design, optimization, in vitro and in vivo evaluation. Drug Deliv. 2013, 21, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.M.; Oh, Y.K.; Choi, H.-G.; Kim, Y.S.; Kim, C.K. Rheological Evaluation of Thermosensitive and Mucoadhesive Vaginal Gels in Physiological Conditions. Int. J. Pharm. 2002, 241, 155–163. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).