Abstract
For the structure and functioning of bio-molecules, metals are important. The main focus of research remains the design and synthesis of novel metal-based complexes and metal ion binding to substances in search of novel medicines. Studies have established the well-defined geometry, thermodynamic stability and excellent coordination power of vanadium in different oxidation states. This paper summarizes the biological activities of vanadium complexes, particularly their anticancer activity. Future multidisciplinary research and analysis focused on comprehending the biochemistry of vanadium complexes with different ligands is required.
1. Introduction
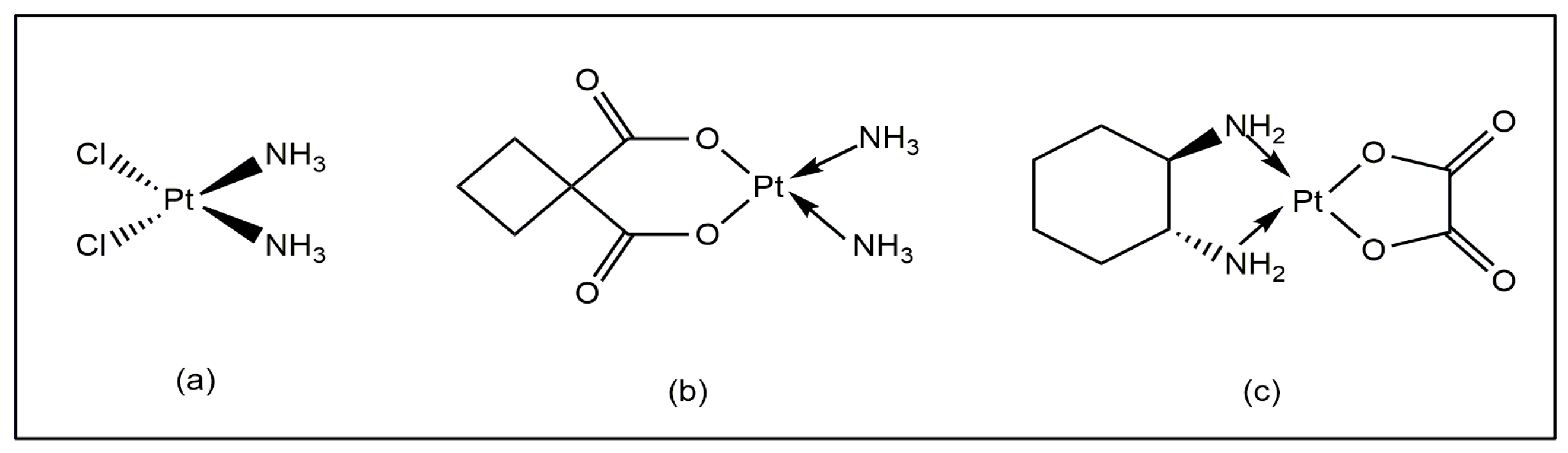
Metals ions control a wide range of important biological processes, with remarkable sensitivity and selectivity. In the sixteenth century, several metal ions were implicated in the prevention and treatment of human cancer [1]. Metal ions and their ligands exhibit a wide range of physiochemical properties, redox states, coordination numbers and geometries, resulting in a variety of reactivities, which are important tools for research in this area. Inorganic biochemistry provides interesting opportunities for the development of effective medicinal drugs [2,3]. It is important to mention that cisplatin (Figure 1a) has been successfully used in medicine to treat several types of tumors [4], but it has several adverse effects [5]. New platinum-based drugs like carboplatin and oxaliplatin (Figure 1b,c) helped, to some extent, to mitigate the drawbacks of cisplatin [6,7]. The recent advancements in medicine have established a wide variety of metal compounds with low toxicity and side effects for treating tumors [8,9]. There have been several reports regarding the wide-ranging uses of vanadium complexes as potential therapeutic agents with low toxicity [10].
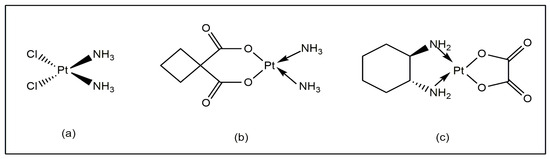
Figure 1.
(a) Cisplatin, (b) carboplatin, (c) and oxaliplatin.
2. Anticancer Activity of Vanadium Complexes
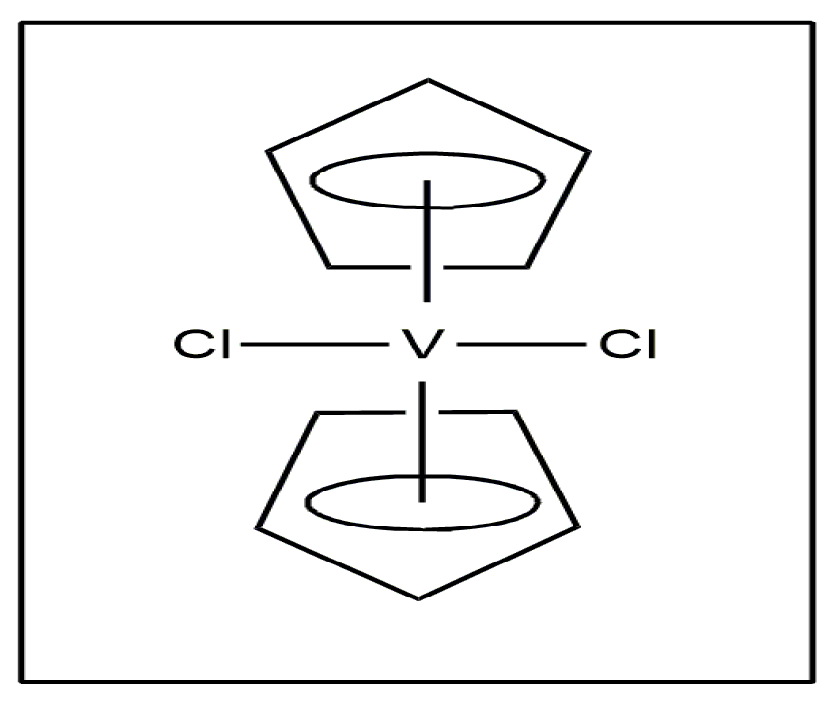
Vanadium exists in many forms, ranging simple inorganic salts to more complicated coordination complexes with both organic and inorganic ligands [11,12]. Vanadocene, a vanadium-based drug, exhibited anticancer activity, and it is a member of metallocene [13]. The first vanadocene sample to exhibit an important preclinical result was vanadocene dichloride (VCp2Cl2) (Figure 2) [14].
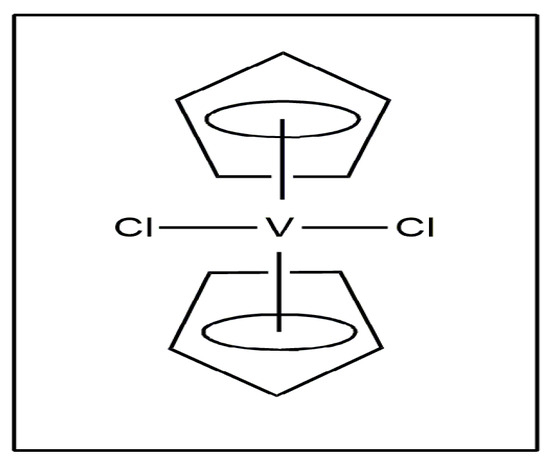
Figure 2.
Vanadocene dichloride.
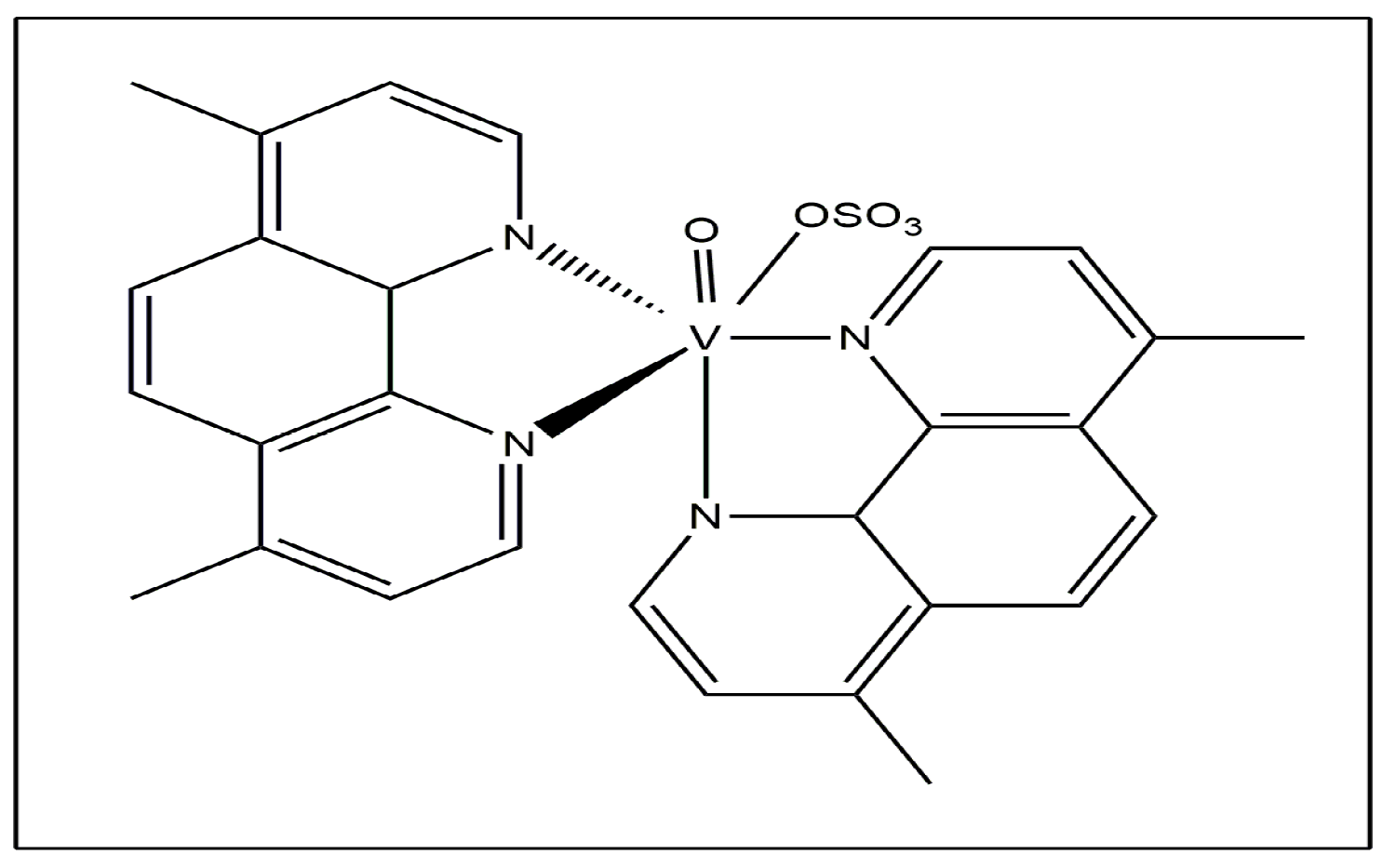
Recent research into speciation has demonstrated that [VCp2Cl2] evolves into [VCp2(OH)2] at physiological pH, and the two OH- ions may be displaced by carbonate, oxalate, phosphate and lactate to form the adducts [VCp2(CO)3], [VCp2(ox)], [VCp2(HPO4)] and [VCp2(lacH)(−1)] with these ligands [15]. The methyl- and methoxy-substituted vanadocene dichlorides exhibited anticancer activity against T-lymphocytic leukemia cells using MOLT-4 [16]. Oxidovanadium (IV) complexes such as Metvan [bis(4,7-dimethyl-1,10-phenanthroline)sulfato-oxovanadium (IV)] (Figure 3) exhibited anticancer activity.
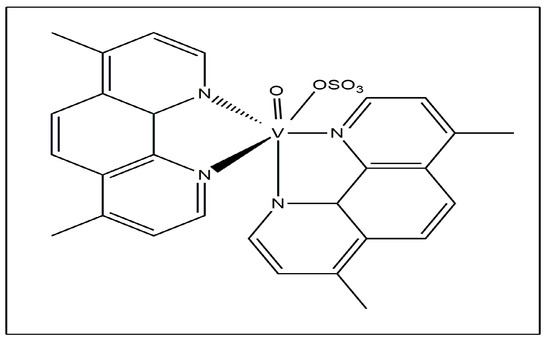
Figure 3.
Metvan.
Several human cancer cell lines, including leukemia cells, solid tumors and multiple myeloma cells present in the ovary, breast, testis and prostrate, are damaged by this complex through apoptosis [17,18,19]. Due to its positive pharmacodynamics properties and low toxicity, it has the potential to be the first vanadium complex used in place of traditional platinum chemotherapy [20]. Many flavonoids, like morin, quercetin, hesperidin, chrysin and silibinin, as well as their oxidovanadium (IV) complexes, have been investigated to reduce the proliferation of both normal (MC3T3E1) and malignant (UMR106) osteoblast cells [21,22,23]. In human osteosarcoma cells, MG-63, oxidovanadium(IV)-silibinin (VOsil) and oxidovanadium(IV)-chrysin (VOchrys) have all been well studied. VOchrys inhibited cell viability in a concentration-dependent manner in human osteosarcoma cells. Additionally, VOchrys had a lower IC50 value of 16 µM compared to values 100 µM for vanadyl cation and chrysin, so it was the most potent anticancer agent in human osteosarcoma cells [24].
Moreover, VOsil reduced the cell viability of the MG-63 cell line in a dose-dependent manner more effectively than vanadyl cation and silibinin. The complex showed concentration effects in both cyto- and genotoxic pathways [25]. Mixed ligands complexes of oxidovanadium(IV) with Schiff base and thiosemicarbazone showed antitumor activity toward several colonic cancer cell lines, like HT-29, HTC-116 and Caco-2, along with non- malignant colon myofibroblasts (CCD18-Co) [26]. Some biologically active vanadium complexes and their anticancer activities with different ligands are illustrated in Table 1.

Table 1.
Anticancer activity of vanadium complexes with different ligands.
3. Probable Mechanistic Action of Vanadium Complexes
3.1. The Warburg Effect: Targeting Tumor Cell Metabolism
Oxidovanadium compounds have been reported to arrest the G0/G1 phase cell cycle and lower causing mitochondrial membrane depolarization in the human hepatoma cell lines HUH-7, HepG2 and BEL-7402 [31]. In another investigation, the metabolism of cancer cells can be modified by vanadium [32]. Cancer cells, compared to normal cell metabolism, upregulated glycolysis and glucose absorption, which caused an increase in the formation of glycolytic metabolites and pyruvate. In cancer cells, glycolysis is uncoupled from the mitochondrial tricarboxylic acid (TCA) cycle and oxidative phosphorylation. As a result, numerous pyruvates produced during glycolysis were shifted toward lactate fermentation compared to the mitochondrial oxidative metabolism. This metabolic process is known as the “Warburg effect”, as it was first discovered by Otto Warburg. Warburg phenotype is a typical tumor-related trait [33].
3.2. Vanadium Compounds and Formation of Reactive Oxygen Species
A potent anticancer treatment involves the redox balance because cancer cells are highly susceptible to redox susceptibility, including hypoxia [34]. Complexes of metals can directly or indirectly influence cellular redox balance by reducing/oxidizing metal or ligand centers, as well as interactions with biomolecules in redox systems [35]. Only cancer cells are affected by the redox activation of metal complexes, thus reducing the adverse impacts. It has been demonstrated that vanadium complexes produce ROS (OH. And O2.), both in the solvated ions and gas phase [36]. Anticancer activity against thyroid papillary carcinoma has been demonstrated by vanadium complexes [37]. At low concentrations, orthovanadate induced tumor suppression, which increased RET/PTC1 tyrosine 451 phosphorylation and activated the Mtor/S6R member of the P13K/AKT signaling route via apoptosis, which included the loss of mitochondrial membrane potential, ROS generation, DNA fragmentation and the activation of caspase-3 [38]. Vanadium complexes also induced ROS-mediated apoptosis in A549 lung adenocarcinoma and the MCF-7 human breast cancer cell line by reducing metalloproteinase-2(MMP-2) and H-ras activation [39].
3.3. Transforming Growth Factor-β (TGFβ) Epithelial-to-Mesenchymal Transition (EMT) Signaling Path
Several studies indicated that vanadium prevents tumor cells spread via lowering production of MMP-2 or induced ROS-dependent apoptosis [40]. Using human breast cancer MDA-MB-231 epithelial cell cultures and lung cancer A549, Petanidis et al. first documented the detrimental impact of vanadium on (TGF-β)-mediated EMT and the subsequent downmodulation of tumor stem cell signaling. Additionally, they suggested that vanadium and carboplatin, working in combination, arrest the G0/G1 cell cycle and sensitize tumor cells to carboplatin-induced death. This information is used to target cancer stem cell-mediated metastasis and cancer cell metabolism in chemoresistant cells [41].
3.4. Focal Adhesion Kinase (FAK) Signaling Path
FAK is essential for cancer cell adhesion, angiogenesis, survival, metastatic growth, and motility [42]. Recently, it was shown that oxidovanadium(IV)-clioquinol (VO(CQ)2) and VOchrys complexes inhibit FAK, thus decreasing the proliferation of human osteosarcoma cells [43,44]. The results indicated that VO(CQ)2 is situated in the kinase domain stimulation loop and interacts with proteins in the ATP site of binding. VO(CQ)2 showed that the upmodulation of Tyr576 and Tyr577 sites at 2.5 µM and the activation of Tyr576 and Tyr577 at 10 µM reduced 14-fold [45]. In a related study, researchers discovered that VOchrys upregulated the phosphorylation of the Tyr577 site, while it downregulated Tyr397 [43]. The most active site for the autocatalytic action of FAK is the Tyr397 site, which is responsible for the phosphorylation of tyrosine [42]. These findings suggest that VOchrys targets the Tyr397 site to inhibit the phosphorylation of FAK [46].
3.5. The Notch-1 Signaling Path
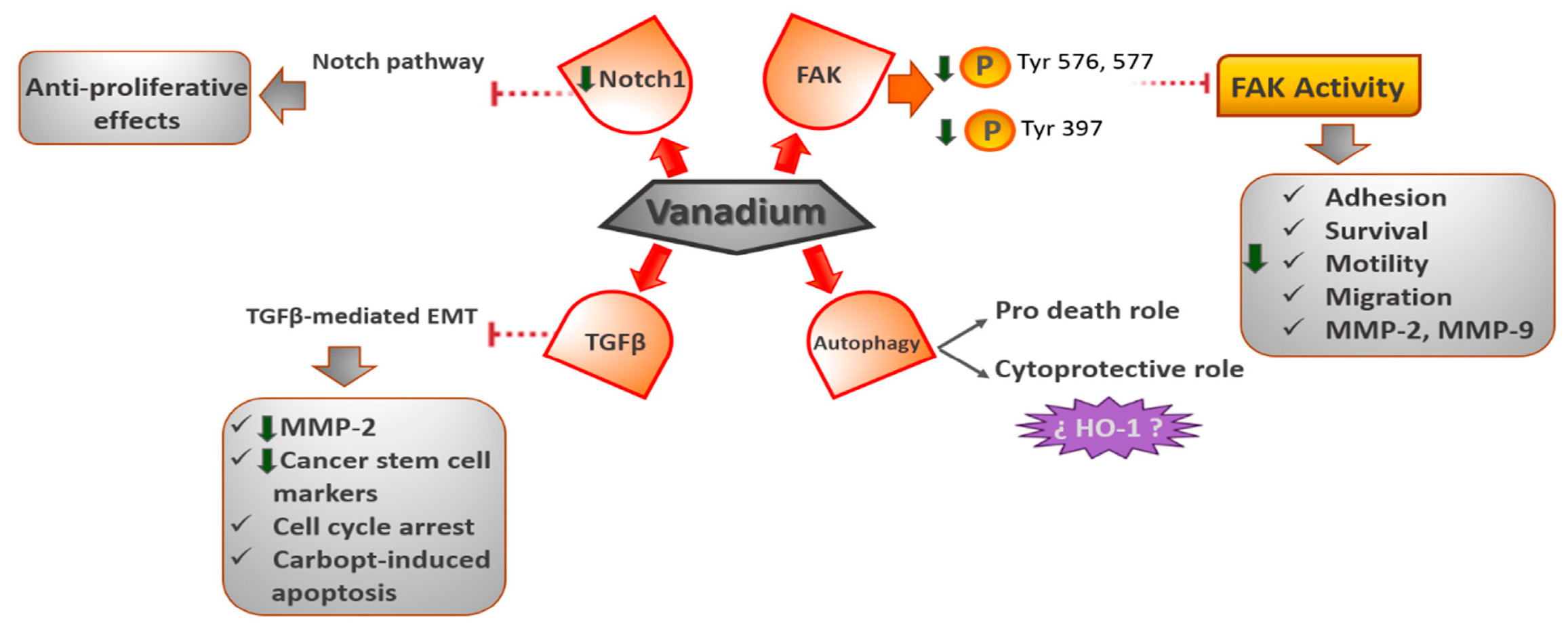
The Notch-1 signaling route is a highly regulated cell signaling mechanism that regulates the development of embryos, as well as disrupting many kinds of tumors, like breast or lung cancer [47,48,49]. Recently, it has been demonstrated that complexes of vanadium inhibit the proliferation of the MDA-MB-231 cell line, which is an example of malignant and triple-negative breast cancer that is resistant to therapy. The [VO(bpy)2Cl]Cl compound (bpy = bipyridyl) increases caspase-3, levels including apoptosis cell death [50]. The researchers also discovered that the Notch-1 pathway was inhibited via a reduction in the production of the Notch-1 gene [47]. Furthermore, Y-cell acute lymphoblastic leukemia in animal models and cultured cells has been shown to exhibit antiproliferative effects when Notch-1 signaling is inactivated [49] (Figure 4) [40] [open access].
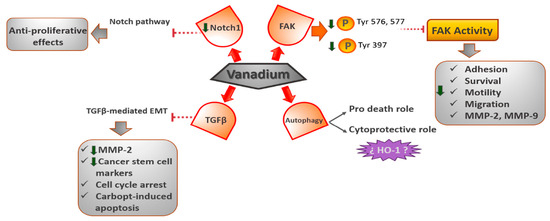
Figure 4.
Downregulation of proteins induced by vanadium complexes for cell survival and death.
4. Conclusions
This paper summarized the anticancer activity of vanadium complexes with different ligands. Studies have established well defined geometry, thermodynamic stability and excellent coordination power of vanadium in different oxidation states. Multidisciplinary research focused on comprehending the biochemistry of vanadium complexes with different ligands as well as synthesizing novel complexes which have low toxicity, better solubility and bio-availability.
Author Contributions
Conceptualization, K.H. and S.J.; resources, S.J.; data curation, S., K.H. and S.J.; writing-original draft preparation, S., K.H., S.G., A.S. and S.J.; writing-review and editing, S. and S.J.; visualization, S., K.H., S.G., A.S. and S.J.; supervision, S.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Frezza, M.; Hindo, S.; Chen, D.; Davenport, A.; Schmitt, S.; Tomco, D.; Dou, Q.P. Novel metals and metal complexes as platforms for cancer therapy. Curr. Pharm. Des. 2010, 16, 1813–1825. [Google Scholar] [CrossRef]
- Orvig, C.; Abrams, M.J. Medicinal inorganic chemistry: Introduction. Chem. Rev. 1999, 99, 2201–2204. [Google Scholar] [CrossRef]
- Thompson, K.H.; Orvig, C.; Sadler, P.J.; Abrams, M.J.; Murrer, B.A.; Farrell, N.; Thompson, K.H.; Bull, S.R. Metal complexes in medicinal chemistry: New vistas and challenges in drug design. Dalton Trans. 2006, 36, 761–764. [Google Scholar] [CrossRef]
- Hambley, T.W. Developing new metal-based therapeutics: Challenges and opportunities. Dalton Trans. 2007, 21, 4929–4937. [Google Scholar] [CrossRef]
- Jung, Y.; Lippard, S.J. Direct cellular responses to platinum-induced DNA damage. Chem. Rev. 2007, 107, 1387–1407. [Google Scholar] [CrossRef]
- Satya; Hashmi, K.; Gupta, S.; Singh, N.; Khan, T.; Joshi, S. Nanofabrication of Metals and Their Compounds for Effective Medicinal and Environmental Applications (A Review). Russ. J. Gen. Chem. 2023, 93, 635–665. [Google Scholar] [CrossRef]
- van Zutphen, S.; Reedijk, J. Targeting platinum anti-tumour drugs: Overview of strategies employed to reduce systemic toxicity. Coord. Chem. Rev. 2005, 249, 2845–2853. [Google Scholar] [CrossRef]
- Koepf-Maier, P.; Koepf, H. Non-platinum group metal antitumor agents. History, current status, and perspectives. Chem. Rev. 1987, 87, 1137–1152. [Google Scholar] [CrossRef]
- Ott, I.; Gust, R. Non platinum metal complexes as anticancer drugs. Arch. Pharm. 2007, 340, 117–126. [Google Scholar] [CrossRef]
- Pessoa, J.C.; Etcheverry, S.; Gambino, D. Vanadium compounds in medicine, Coord. Chem. Rev. 2015, 301–302, 24–48. [Google Scholar] [CrossRef]
- Rehder, D. Vanadium; Its Role for Humans. In Interrelations between Essential Metal Ions and Human Diseases; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Bishayee, A.; Waghray, A.; Patel, M.A.; Chatterjee, M. Vanadium in the detection, prevention and treatment of cancer: The in vivo evidence. Cancer Lett. 2010, 294, 1–12. [Google Scholar] [CrossRef]
- Navara, C.S.; Benyumov, A.; Vassilev, A.; Narla, R.K.; Ghosh, P.; Uckun, F.M. Vanadocenes as potent antiproliferative agents disrupting mitotic spindle formation in cancer cells. Anticancer. Drugs 2001, 12, 369–376. [Google Scholar] [CrossRef]
- Harding, M.M.; Mokdsi, G. Antitumour metallocenes: Structure-activity studies and interactions with biomolecule. Curr. Med. Chem. 2000, 7, 1289–1303. [Google Scholar] [CrossRef]
- Sanna, D.; Ugone, V.; Micera, G.; Pivetta, T.; Valletta, E.; Garribba, E. Speciation of the potential antitumor agent vanadocene dichloride in the blood plasma and model systems. Inorg. Chem. 2015, 54, 8237–8250. [Google Scholar] [CrossRef]
- Aubrecht, J.; Narla, R.K.; Ghosh, P.; Stanek, J.; Uckun, F.M. Molecular genotoxicity profiles of apoptosis-inducing vanadocene complexes. Toxicol. Appl. Pharmacol. 1999, 154, 228–235. [Google Scholar] [CrossRef]
- Evangelou, A.M. Vanadium in cancer treatment. Crit. Rev. Oncol. Hematol. 2002, 42, 249–265. [Google Scholar] [CrossRef]
- Narla, R.K.; Dong, Y.; Klis, D.; Uckun, F.M. Bis(4,7- dimethyl-1,10-phenanthroline) sulfatooxovanadium(I.V.) as a novel antileukemic agent with matrix metalloproteinase inhibitory activity. Clin. Cancer Res. 2001, 7, 1094–1101. [Google Scholar]
- Dong, Y.; Narla, R.K.; Sudbeck, E.; Uckun, F.M. Synthesis, X-ray structure, and anti-leukemic activity of oxovanadium(IV) complexes. J. Inorg. Biochem. 2000, 78, 321–330. [Google Scholar] [CrossRef]
- D’Cruz, O.J.; Uckun, F.M. Metvan: A novel oxovanadium(IV) complex with broad spectrum anticancer activity. Expert Opin. Investig. Drugs 2002, 11, 1829–1836. [Google Scholar] [CrossRef]
- Naso, L.; Ferrer, E.G.; Lezama, L.; Rojo, T.; Etcheverry, S.B.; Williams, P. Role of oxidative stress in the antitumoral action of a new vanadyl(IV) complex with the flavonoid chrysin in two osteoblast cell lines: Relationship with the radical scavenger activity. J. Biol. Inorg. Chem. 2010, 15, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Naso, L.G.; Ferrer, E.G.; Butenko, N.; Cavaco, I.; Lezama, L.; Rojo, T.; Etcheverry, S.B.; Williams, P.A. Antioxidant, DNA cleavage, and cellular effects of silibinin and a new oxovanadium(IV)/silibinin complex. J. Biol. Inorg. Chem. 2011, 16, 653–668. [Google Scholar] [CrossRef] [PubMed]
- Naso, L.G.; Lezama, L.; Rojo, T.; Etcheverry, S.B.; Valcarcel, M.; Roura, M.; Salado, C.; Ferrer, E.G.; Williams, P.A. Biological evaluation of morin and its new oxovanadium(IV) complex as antioxidant and specific anti-cancer agents. Chem. Biol. Interact. 2013, 206, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Leon, I.E.; Di Virgilio, A.L.; Porro, V.; Muglia, C.I.; Naso, L.G.; Williams, P.A.; Bollati-Fogolin, M.; Etcheverry, S.B. Antitumor properties of a vanadyl(IV) complex with the flavonoid chrysin [VO(chrysin)2EtOH]2 in a human osteosarcoma model: The role of oxidative stress and apoptosis. Dalton Trans. 2013, 42, 11868–11880. [Google Scholar] [CrossRef] [PubMed]
- Leon, I.E.; Porro, V.; Di Virgilio, A.L.; Naso, L.G.; Williams, P.A.; Bollati-Fogolín, M.; Etcheverry, S.B. Antiproliferative and apoptosis-inducing activity of an oxidovanadium(IV) complex with the flavonoid silibinin against osteosarcoma cells. JBIC J. Biol. Inorg. Chem. 2014, 19, 59–74. [Google Scholar] [CrossRef]
- Lewis, N.A.; Liu, F.; Seymour, L.; Magnusen, A.; Erves, T.R.; Arca, J.F.; Beckford, F.A.; Venkatraman, R.; González-Sarrías, A.; Fronczek, F.R.; et al. Synthesis, characterization, and preliminary in vitro studies of vanadium(IV) complexes with a Schiff base and thiosemicarbazones as mixed-ligands. Eur. J. Inorg. Chem. 2012, 2012, 664–677. [Google Scholar] [CrossRef] [PubMed]
- Dankhoff, K.; Ahmad, A.; Weber, B.; Biersack, B.; Schobert, R. Anticancer properties of a new non-oxido vanadium (IV) complex with a catechol-modified 3, 3′-diindolylmethane ligand. J. Inorg. Biochem. 2019, 194, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Banerjee, K.; Banerjee, A.; Das, S.; Choudhuri, S.K. Synthesis, characterization and biological evaluation of a novel vanadium complex as a possible anticancer agent. J. Organomet. Chem. 2014, 772, 34–41. [Google Scholar] [CrossRef]
- Mal, S.K.; Chattopadhyay, T.; Fathima, A.; Purohit, C.S.; Kiran, M.S.; Nair, B.U.; Ghosh, R. Synthesis and structural characterization of a vanadium (V)-pyridylbenzimidazole complex: DNA binding and anticancer activity. Polyhedron 2017, 126, 23–27. [Google Scholar] [CrossRef]
- Meshkini, A.; Yazdanparast, R. Chemosensitization of human leukemia K562 cells to taxol by a Vanadium-salen complex. Exp. Mol. Pathol. 2010, 89, 334–342. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Wang, X.S.; Fang, W.; Cai, X.Y.; Li, H.Z.; Mao, J.W.; Jin, X.B.; Bai, Y.L.; Lu, J.Z. In vitro study of the cytotoxicities of two mixed-ligand oxovanadium complexes on human hepatoma cells. Pharmazie 2013, 68, 827–834. [Google Scholar] [CrossRef]
- Kioseoglou, E.; Petanidis, S.; Gabriel, C.; Salifoglou, A. The Chemistry and Biology of Vanadium Compounds in Cancer Therapeutics. Coord. Chem. Rev. 2015, 301–302, 87–105. [Google Scholar] [CrossRef]
- Hashmi, K.; Gupta, S.; Siddique, A.; Khan, T.; Joshi, S. Medicinal Applications of Vanadium Complexes with Schiff Bases. J. Trace Elem. Med. Biol. 2023, 79, 127245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Sadler, P.J. Redox-active metal complexes for anticancer therapy. Eur. J. Inorg. Chem. 2017, 2017, 1541–1548. [Google Scholar] [CrossRef]
- Jungwirth, U.; Kowol, C.R.; Keppler, B.K.; Hartinger, C.G.; Berger, W.; Heffeter, P. Anticancer activity of metal complexes: Involvement of redox processes. Antioxid. Redox Signal. 2011, 15, 1085–1127. [Google Scholar] [CrossRef]
- Soriano-Agueda, L.A.; Ortega-Moo, C.; Garza, J.; Guevara-Garcia, J.A.; Vargas, R. Formation of reactive oxygen species by vanadium complexes. Comput. Theor. Chem. 2016, 1077, 99–105. [Google Scholar] [CrossRef]
- Gonçalves, A.P.; Videira, A.; Soares, P.; M’aximo, V. Orthovanadate-induced cell death in RET/PTC1-harboring cancer cells involves the activation of caspases and altered signaling through PI3K/Akt/mTOR. Life Sci. 2011, 89, 371–377. [Google Scholar] [CrossRef]
- Petanidis, S.; Kioseoglou, E.; Hadzopoulou-Cladaras, M.; Salifoglou, A. Novel ternary vanadium-betaine-peroxido species suppresses H-ras and matrix metalloproteinase-2 expression by increasing reactive oxygen species-mediated apoptosis in cancer cells. Cancer Lett. 2013, 335, 387–396. [Google Scholar] [CrossRef]
- Etcheverry, S.B.; Crans, D.C.; Keramidas, A.D.; Cortizo, A.M. Insulin-mimetic action of vanadium compounds on osteoblast-like cells in culture. Arch. Biochem. Biophys. 1997, 338, 7–14. [Google Scholar] [CrossRef]
- Ferretti, V.A.; León, I.E. An overview of vanadium and cell signaling in potential cancer treatments. Inorganics 2022, 10, 47. [Google Scholar] [CrossRef]
- El-Shafey, E.S.; Elsherbiny, E.S. Possible Selective Cytotoxicity of Vanadium Complex on Breast Cancer Cells Involving Pathophysiological Pathways. Anticancer Agents Med. Chem. 2019, 19, 2130–2139. [Google Scholar] [CrossRef]
- Luo, M.; Fan, H.; Nagy, T.; Wei, H.; Wang, C.; Liu, S.; Guan, J.L. Guan, Mammary epithelialspecific ablation of the focal adhesion kinase suppresses mammary tumorigenesis by affecting mammary cancer stem/progenitor cells. Cancer Res. 2009, 69, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Lón, I.E.; Díez, P.; Etcheverry, S.B.; Fuentes, M. Deciphering the effect of an oxovanadium(iv) complex with the flavonoid chrysin (VOChrys) on intracellular cell signalling pathways in an osteosarcoma cell line. Metallomics 2016, 8, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Balsa, L.M.; Quispe, P.; Baran, E.J.; Lavecchia, M.J.; León, I.E. In silico and in vitro analysis of FAK/MMP signaling axis inhibition by VO-clioquinol in 2D and 3D human osteosarcoma cancer cells. Metallomics 2020, 12, 1931–1940. [Google Scholar] [CrossRef] [PubMed]
- Ciccimaro, E.; Hevko, J.; Blair, I.A. Analysis of phosphorylation sites on focal adhesion kinase using nanospray liquid chromatography/multiple reaction monitoring mass spectrometry. Rapid Commun. Mass Spectrom. 2006, 20, 3681–3692. [Google Scholar] [CrossRef] [PubMed]
- Golubovskaya, V.M. Targeting FAK in human cancer: From finding to first clinical trials. Front. Biosci. 2014, 19, 687–706. [Google Scholar] [CrossRef]
- Lim, J.S.; Ibaseta, A.; Fischer, M.M.; Cancilla, B.; O’Young, G.; Cristea, S.; Luca, V.C.; Yang, D.; Jahchan, N.S.; Hamard, C. Intratumoural heterogeneity generated by Notch signalling promotes small-cell lung cancer. Nature 2017, 545, 360–364. [Google Scholar] [CrossRef]
- Shen, S.; Chen, X.; Hu, X.; Huo, J.; Luo, L.; Zhou, X. Predicting the immune landscape of invasive breast carcinoma based on the novel signature of immune-related lncRNA. Cancer Med. 2021, 10, 6561–6575. [Google Scholar] [CrossRef]
- Moellering, R.E.; Cornejo, M.; Davis, T.N.; Bianco, C.D.; Aster, J.C.; Blacklow, S.C.; Kung, A.L.; Gilliland, D.G.; Verdine, G.L.; Bradner, J.E. Direct inhibition of the NOTCH transcription factor complex. Nature 2009, 462, 182–188. [Google Scholar] [CrossRef]
- Kumar, R.; Juillerat-Jeanneret, L.; Golshayan, D. Notch Antagonists: Potential Modulators of Cancer and Inflammatory Diseases. J. Med. Chem. 2016, 59, 7719–7737. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).