Abstract
The article deals with the removal of 20 selected drugs from drinking water by sorption on granulated activated carbon. Two different sorption materials were used, and the efficiency of removing these micropollutants was compared. Experiments were performed in laboratory conditions with two different values of pH of water (7.8 and 6.5, respectively), at laboratory temperatures and with an identical amount of added sorbent (Filtrasorb 400, WG12). Standard additions were made to drinking water, and the final concentration of 0.44–0.55 µg/L of pharmaceuticals was utilized in the experiments. Samples were taken after 30, 60, 120 and 240 min of contact of sorbent with water. The LC-MS method was used to analyze the selected pharmaceuticals, which was performed at the ALS laboratory in Prague, Czech Republic. The adsorption efficiency of the removal of the given pharmaceuticals from water, as well as the adsorption capacity of the granulated activated carbon for the given pharmaceuticals, depends on amount of time that the water and material were in contact. The adsorption efficiency for two different types of granulated activated carbon ranges from 13 to 90%. Water pH also influences the sorption abilities of granulated activated carbon.
1. Introduction
Over the previous decades, analytical methods have seen progress in determining micropollutants, leading to detection of trace concentrations of pharmaceuticals not only in surface and wastewater, but also in groundwater, and even in drinking water, in concentrations from nanograms to micrograms.
Pharmaceuticals are natural or synthetic substances that are included in the micropollutant group [1,2]. They can travel into surface or groundwater through municipal wastewater, and they can pose a risk to the environment even in low concentrations. Approximately 3000 various substances are used in human medicine in the European Union, such as analgesics, anti-inflammatory drugs, antibiotics, betablockers, lipid regulators, psychoactive drugs and many more. Many pharmaceuticals are also used in veterinary medicine, including antibiotics and anti-inflammatory drugs. After application, pharmaceutical formulae are ejected from the body in their original (unchanged) form, or in the form of metabolites, and enter water environments by various means [3]. From the environmental point of view, the biggest concern when using pharmaceutical products is their persistence and critical biological activity.
Residues of pharmaceutical products may enter the environment during production, usage, and liquidation. Medicinal products enter the environment mainly through runoff from municipal wastewater treatment plants, wastewater from manufacturing-industrial plants, wastewater from hospitals, application of sewage sludge to fields, veterinary medicine, application of animal manure, leachate from landfills, improper disposal of expired pharmaceuticals, improper disposal of unused medicines and contaminated waste [4].
Pharmaceuticals that are not removed in the wastewater treatment process are discharged into recipients, which leads to the contamination of rivers, lakes, and sometimes even underground and drinking water. According to studies published to date, the concentrations of pharmaceuticals in surface water and groundwater, which have been polluted by discharged wastewater from sewage treatment plants, are lower than 0.1 μg/L, and if they occur in drinking water, their concentrations are lower than 0.05 μg/L [5].
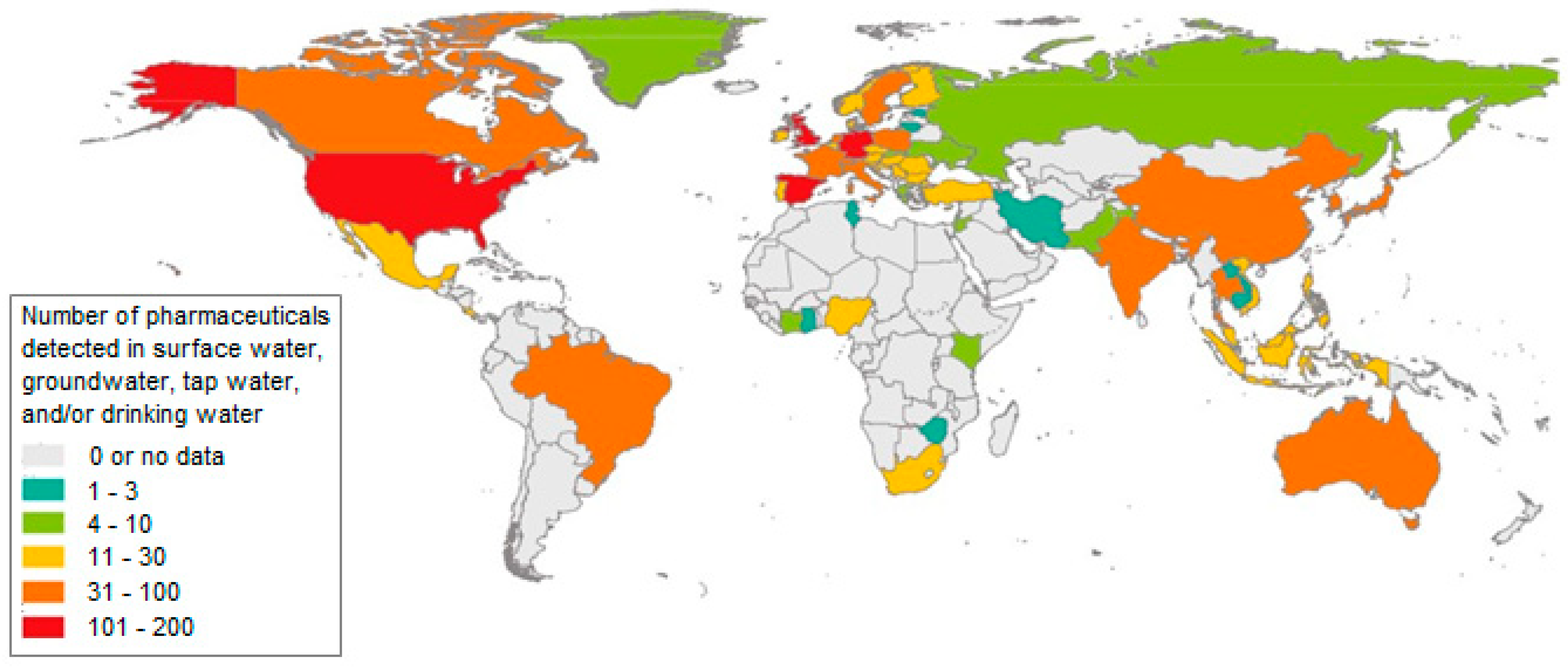
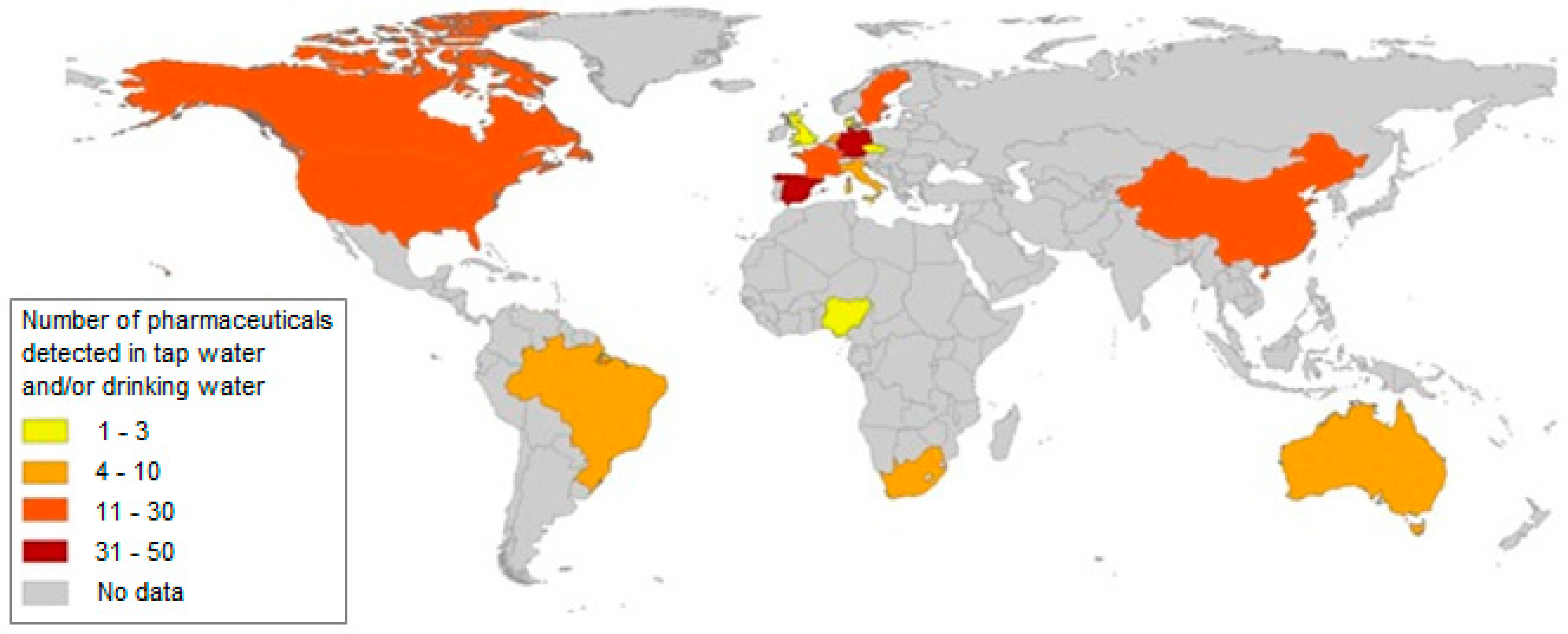
Figure 1 and Figure 2 show the number of pharmaceuticals determined in surface, underground, tap and drinking water.
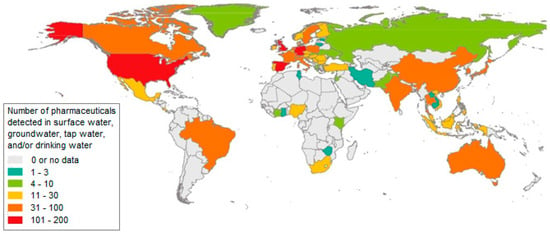
Figure 1.
The number of pharmaceuticals determined in surface, underground, tap and drinking water [6].
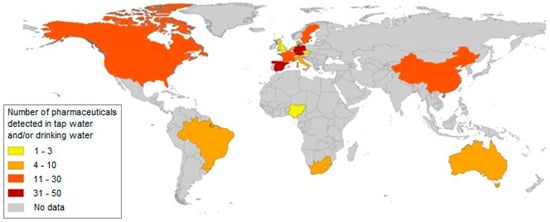
Figure 2.
The number of pharmaceuticals determined in tap and drinking water [6].
On the basis of many published works, including monitoring and studies that dealt with the issue of drugs and their metabolites in water, it has been found that the pollution of water, whether surface, underground or, to a lesser extent, drinking water, by these micropollutants is a worldwide problem. Based on the study of the currently available literature, comprising more than 1000 works, a database was made, which showed that drugs or their transformation products were detected in the environment of 71 countries covering all continents [6].
Pharmaceutical products and their metabolites were also found in Slovak surface waters, and monitoring results indicated the frequent occurrence of the following pharmaceuticals: valsartan, venlafaxine, telmisartan, metoprolol, tramadol, clindamycin, erythromycin, carbamazepine and diclofenac. And some of them were even found in the waters of the High Tatras lakes [7].
1.1. Methods of Removal of Pharmaceuticals from Water
Pharmaceuticals are a diverse group of chemicals with various physical and chemical properties. The removal efficiency depends on their physical and chemical characteristics (e.g., hydrophobicity) and their reactivity to various removal processes, drug concentration, water temperature and hydraulic conditions. Therefore, treatment processes can only achieve a certain level of removal depending on the pharmaceutical product and used technology. These processes, such as reverse osmosis, ozonation and advanced oxidation technologies, can generally achieve higher drug removal rates compared to classical processes [8,9,10].
According to the available literature, the effectiveness of classical treatment of drinking water is low, usually up to 30% [5]. The literature [11] reports interesting results of the removal of some drugs (diclofenac, ibuprofen, bezafibrate, carbamazepine and sulfamethoxazole) from water by chemical coagulation. Based on the results of drug removal from water, which were achieved by coagulation, experiments were done with two types of water: MilliQ water and water reservoir, containing humic acids. Iron (pH 4,5) and aluminum (pH 6) salts were used as coagulants, and HPLC and UV detection were used to determine drugs in the monitored water samples. By coagulating MilliQ water with ferric sulfate, the removal efficiency reached less than 10% with the exception of diclofenac, which was removed with an efficiency of 66%. When using aluminum sulfate to coagulate water from the reservoir, the results were similar, i.e., less than 10% removal efficiency with the exception of diclofenac, which was removed by 30%.
In the presence of dissolved humic substances, diclofenac, as well as ibuprofen and bezafibrate, can be removed by coagulation with iron (III) sulfate with a maximum efficiency of 77% for diclofenac, 50% for ibuprofen and 36% for bezafibrate. A high amount of high molecular weight NOM improved the removal of ionizable drugs. The efficiency of removing non-ionizable compounds of carbamazepine and sulfamethoxazole by coagulation was very low. In the case where water had a high humic substance content, low pH values and iron coagulant, the effectiveness was higher, but basically, based on the results achieved with coagulation, removing drugs from water in this way is not effective.
Based on the research devoted to the removal of drugs from water, it follows that more suitable methods are the use of slow sand filtration and adsorption on granular activated carbon filters or the addition of powdered activated carbon to the water [5].
Adsorption on GAC is used in the water treatment processes, and it removes pharmaceuticals from water with higher efficiency than coagulation. Their efficiency is high, in some cases up to 98%, but this is not true in all situations. The efficiency depends on the nature of the organic substance being removed, the pH of the water and the presence of substances that affect sorption (e.g., humic substances, turbidity).
In the research carried out at Brno University of Technology (BUT), the effectiveness of removing ibuprofen (at a concentration of 1.02 µg/L) [12] and diclofenac (at a concentration of 1.28 µg/L) [13] from drinking water using the sorbents Filtrasorb F100, Bayoxide E33 and GEH was monitored. The measurements took place in a column with an internal diameter of 4.4 cm and a filling height of 70–80 cm, sampling 1 to 6 min after the start of the experiment. At the same time, the change in water quality influenced by the material used (pH, temperature and turbidity) was monitored. Ibuprofen removal efficiency ranged from 71 to 88% for F100, 85–92% for Bayoxide E33 and 81–85% for GEH in the first two minutes, then desorption occurred. In case of diclofenac, the GEH material achieved the best adsorption efficiency (99%), followed by Filtrasorb F100 (92–99%) and Bayoxide E33 (only 24–50%), depending on the sampling time (from 1 to 6 min).
Groundwater used for drinking purposes is water that is, in most cases, equal to the quality of drinking water. When treatment of such water is necessary, it is due to increased iron, manganese and heavy metal contents; the concentration of these materials must be reduced to values corresponding to drinking water. Often, it is sufficient to use disinfection. Disinfectants have demonstrated the ability to reduce drug concentrations in water; as stated in the WHO document [5], chlorine disinfectant can remove up to 50% of monitored drugs from water. The chlorine compound chloramine was also monitored, but here, the antibiotic removal efficiency was very low. Chlorine dioxide and ozone also show high removal efficiency [14,15,16].
Currently, some of the most effective ways to remove drugs from water are membrane processes. In the case of using membrane processes, the efficiency is influenced not only by the properties and nature of the drugs, but also by the properties of the membranes. The removal efficiency can reach up to 90% [17].
At present, pharmaceutical removal strategies can generally be divided into physical, chemical and biological methods. The advanced oxidation processes (AOPs), enzyme degradation, carbonaceous material Biochar, carbon nanotubes, nanoparticles, metals, biosorbents, granular activated carbons, resins, etc. may be an effective method for treating serious environmental pollution [18,19,20,21,22,23,24,25]. Of these methods, some are already being used in practical application to reduce the danger of the presence of drugs that threaten not only the aquatic environment, but also human health.
1.2. Pharmaceuticals Used in This Research
The supplied standard mixture contained the following types of pharmaceuticals: anti-inflammatory agents (diclofenac, indomethacin, ketoprofen, naproxen, paracetamol), antibiotics (metronidazole, sulfamethazine, sulfamethoxazole, trimethoprim), contrast agents (iomeprol, iopamidol, iopromide), circulatory system agents (valsartan, warfarin, hydrochlorothiazide) and others (bezafibrate, fluoxetine, ifosfamide, carbamazepine, caffeine).
For example, carbamazepine is an anticonvulsant drug used primarily to treat epilepsy and neuropathic pain. It is used to treat schizophrenia, along with other drugs, and is a second-line agent in bipolar disorder. Caffeine is a central nervous system stimulant from the methylxanthine group. It is the most widespread psychoactive drug in the world. Caffeine concentrations in surface waters were detected in the range of 0.1–6.9 µg/L.
Bezafibrate is a drug used as a lipid-lowering agent to treat hyperlipidemia. Fluoxetine is a drug used mainly in the treatment of depression. Ifosfamide is a chemotherapy drug used to treat many types of cancer. These include soft tissue sarcoma, osteosarcoma, bladder cancer, small cell lung cancer, cervical cancer and ovarian cancer.
Diclofenac is one of the most commonly prescribed medications, used to treat pain and inflammatory diseases. It is available in the form of a variety of medicinal, some of them are free of the counter without a prescription, but only at certain doses.
The occurrence of pharmaceuticals, their metabolites and transformation products in the environment has become the subject of research from the point of view of toxicity, monitoring and analytical determination. Various pharmaceuticals have been detected in many samples of environmental components around the world. Their occurrence has been recorded in runoff and sludge from wastewater treatment plants, surface water, seawater, groundwater, soil and sediments as well as in drinking water.
Therefore, the aim of our research was to verify the effectiveness of granular activated carbon in removing selected drugs from water to compare two different GACs, as well as the effect of water pH on their effectiveness.
2. Materials and Methods
The pharmaceutical standard was purchased from the ALS Czech Republic company in Prague, which also provided us with sample boxes and determined the drugs in the samples. The standard contained 20 different drugs. Granulated activated carbon WG12 (manufactured by Gryfskand Co., Hajnówka, Poland) was supplied by Envi-Pur, LtD. and Filtrasorb F400 (manufactured by Calgon Carbon Co., and delivered by Chemviron Carbon, Feluy, Belgium) by Jako LtD., both from the Czech Republic. The basic properties of the used granular activated carbon are summarized in Table 1.

Table 1.
Properties of activated carbon WG12 and F400.
A model solution with a drug content in the range from 0.44 µg/L to 0.55 µg/L was obtained by adding 50 mL of the drug mixture standard to 5 L of drinking water. The model water sample prepared in this way was divided into Erlenmayer flasks; 400 mL of model water was added to each sample, and 400 mg of granular activated carbon (Filtrasorb F400 and WG12) was added to the samples that were prepared in this way. The volume of model water together with activated carbon was mixed for 4 h using an OHAUS orbital shaker (OHAUS Europe GmbH, Nänikon, Switzerland) at 400 rpm. During the mixing, water samples were taken from the flasks at time intervals of 0, 30, 60, 120 and 240 min. The samples were collected in glass vials (40 mL) which contained a preservative (0.32 mL of 1% sodium thiosulfate).
The collected water samples (10 mL) after centrifugation and microfiltration were injected into a liquid chromatography device (Acquity UPLC I-Class, Waters Co., Milford, CT, USA) connected to a mass spectrometer (XEVO TQ-XS, Waters Co., Milford, CT, USA). The compounds were separated by a chromatographic column (InfinityLab Poroshell 10 EC-C18 (3.0 × 150 mm; 2.7 µm, Agilent Technologies, Santa Clara, CA, USA) mobile phase consisted of 0.01% HCOOH in water (A) and MeOH (B). The obtained data were evaluated with the Tar-getLynx quantification software. Quantification of analytes was conducted using the method of isotopically labeled internal standards addition.
3. Results and Discussion
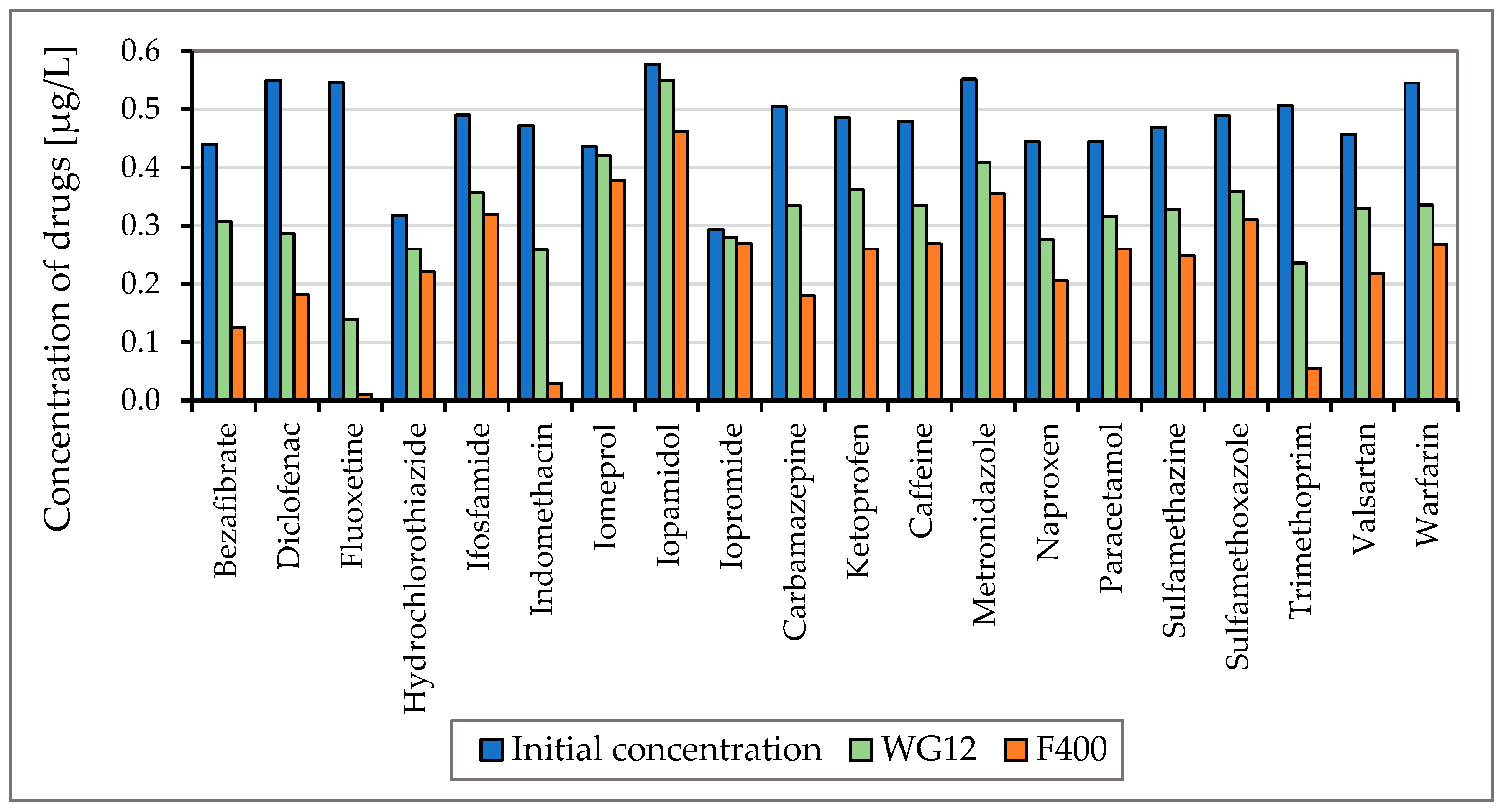
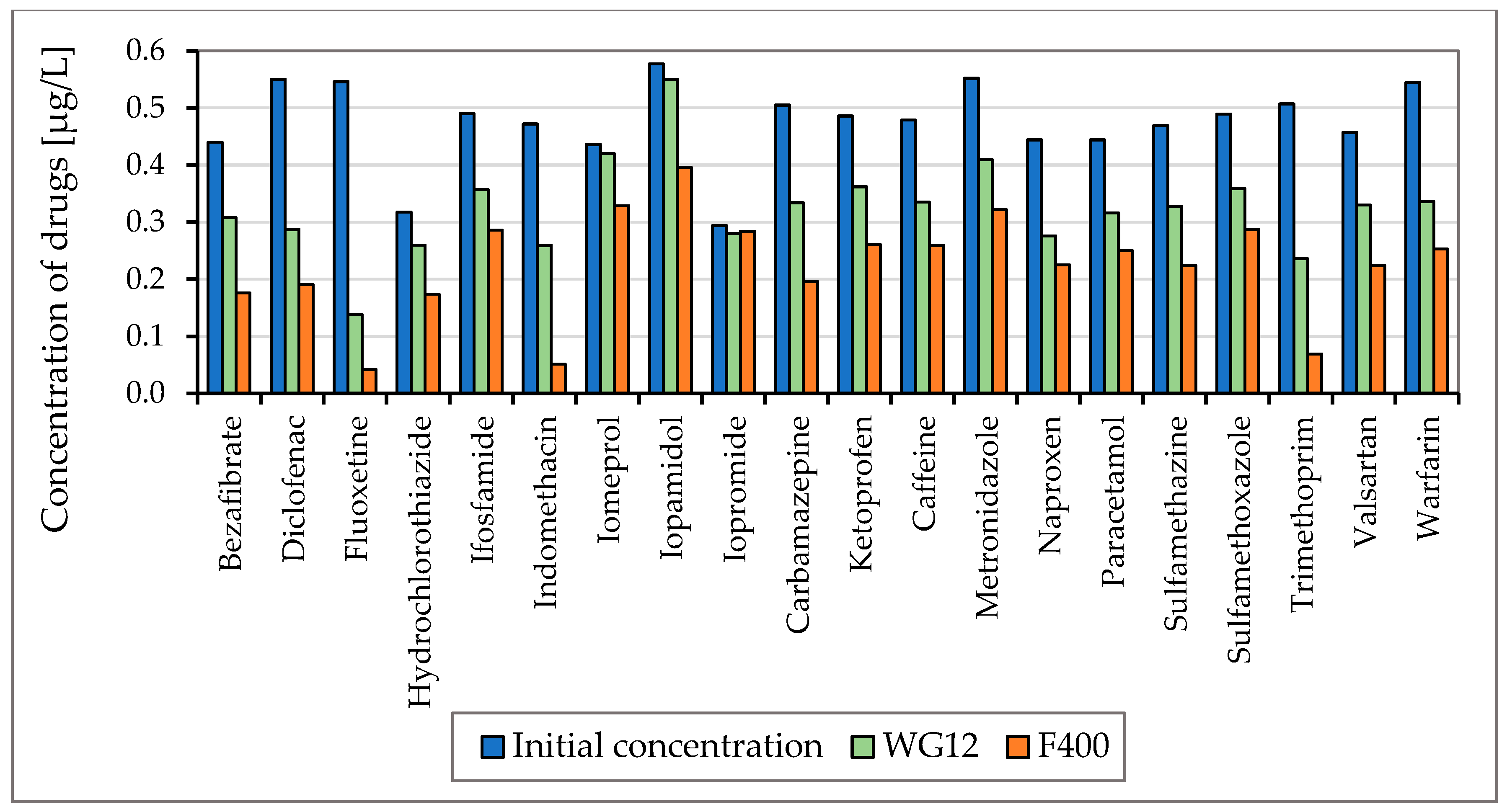
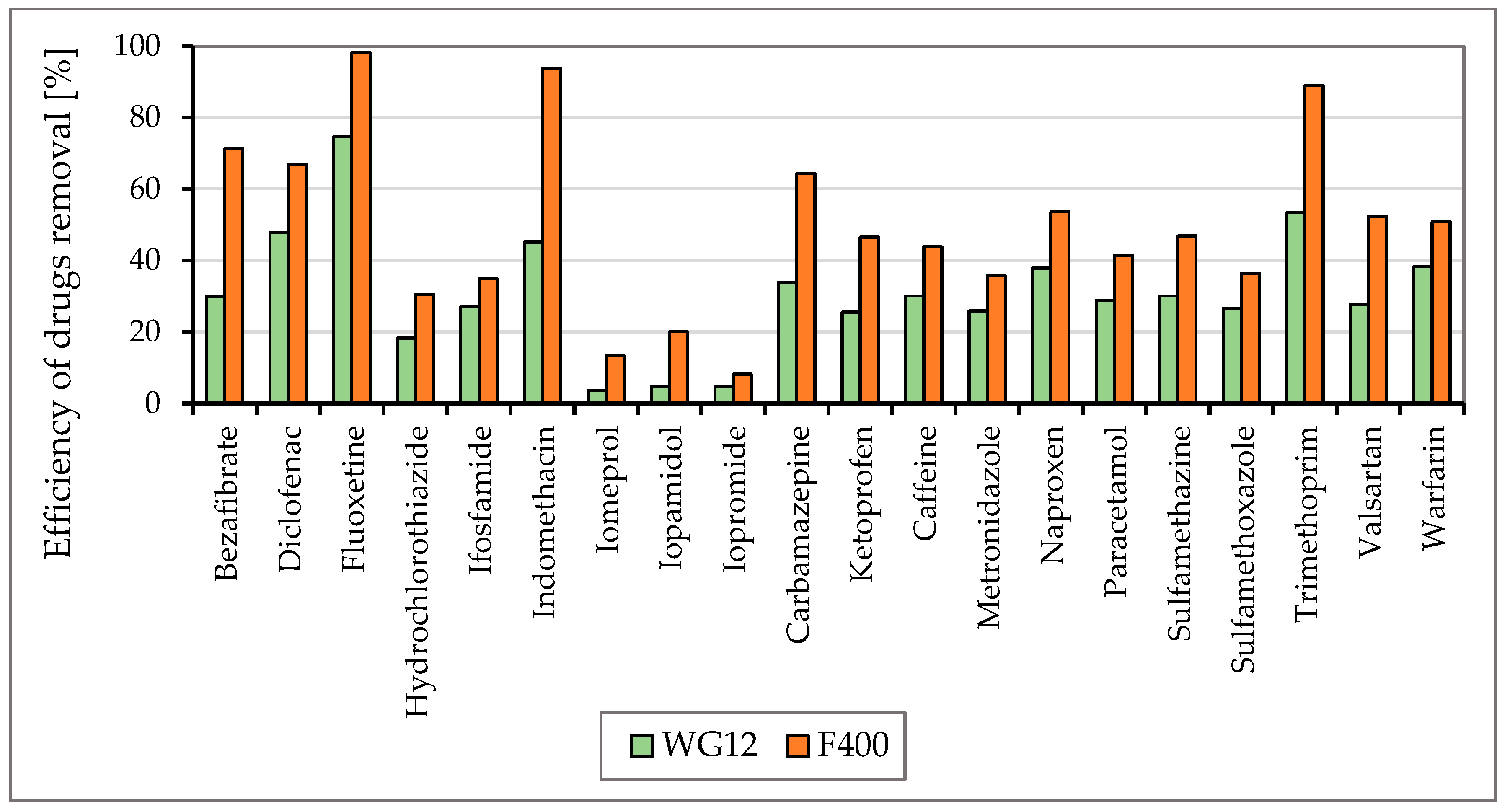
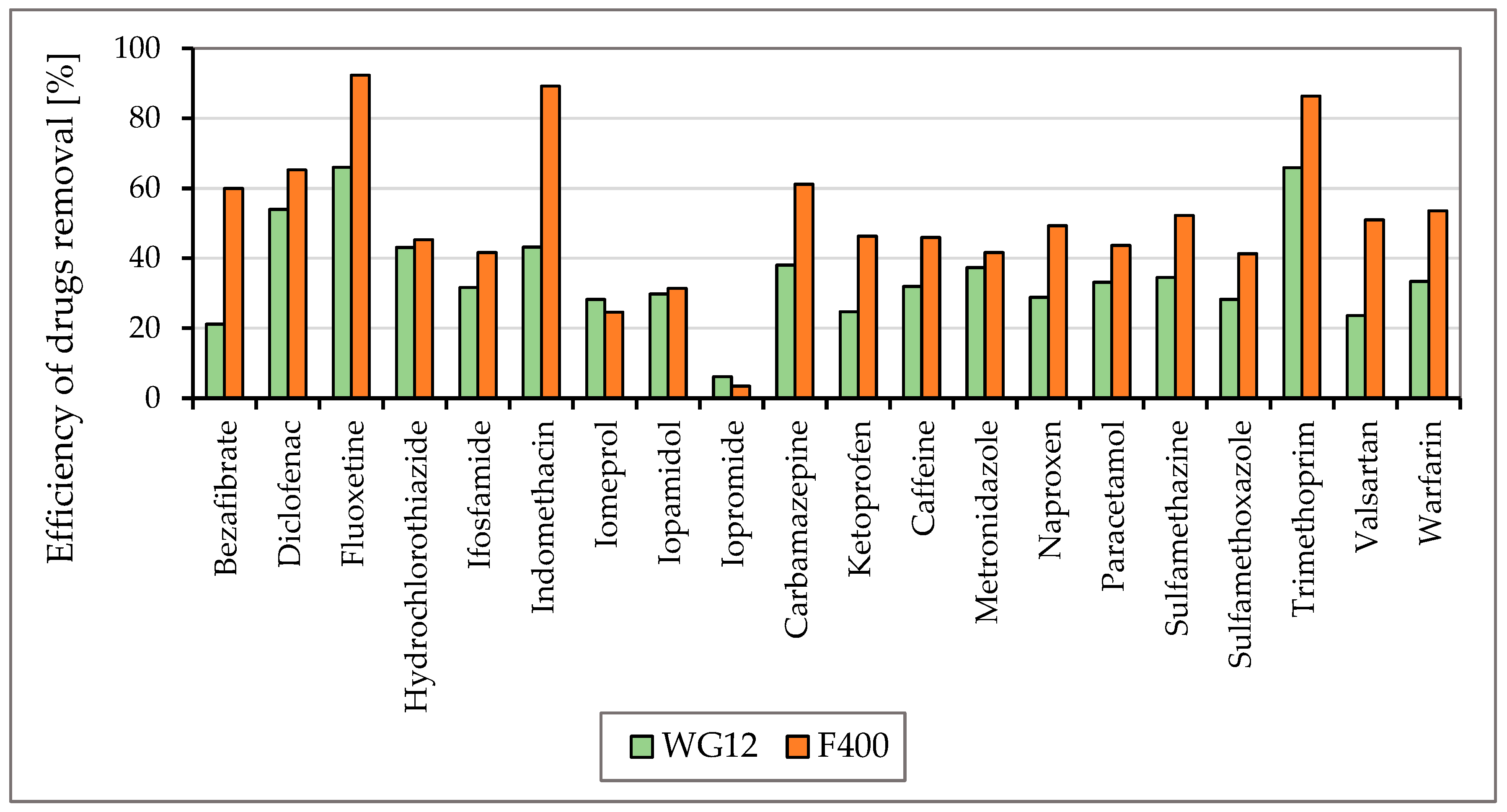
To monitor the efficiency of the removal of drugs listed in Figure 3 and Figure 4, two types of activated carbon, Filtrasorb F400 and WG12, were used in static tests. The efficiency of the sorbent materials was monitored at pH values of 7.8 and 6.5. The concentration of drugs in the water at the beginning of the tests was in the range of 0.44–0.55 µg/L, and the contact time of the model water with activated carbon varied in 30 min intervals up to four hours.
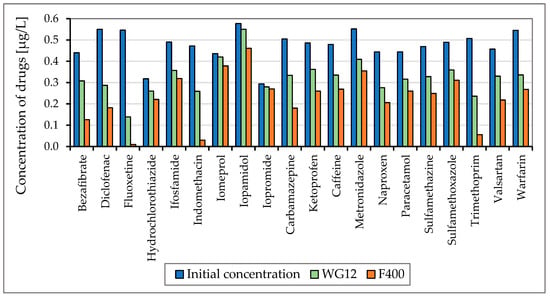
Figure 3.
Concentration of drugs (µg/L) before and after 60 min adsorption with WG12 and F400 at the pH value 7.8.
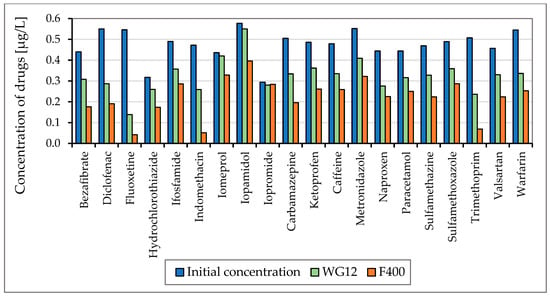
Figure 4.
Concentration of drugs (µg/L) before and after 60 min adsorption with WG12 and F400 at the pH value 6.5.
The adsorption efficiency (in %) and immediate adsorption capacity (in µg/g) of activated carbons WG12 and F400 were calculated for the individual pharmaceuticals depending on the water–material contact time on the base of the measured concentrations of the individual organic compounds [25]. The following formulas were used:
where at is the immediate adsorption capacity in µg/g, η is the adsorption efficiency [%], co is the concentration of pesticides before the adsorption, cm is the concentration of drugs after the adsorption at the time t [µg/L], V is the volume of water solution of 0.4 L and m is the weight of sorption material, 0.4 g.
Figure 5 and Figure 6 show the efficiencies for each granular activated carbon calculated for 60 min of water contact time with the material used at the different pH of water.
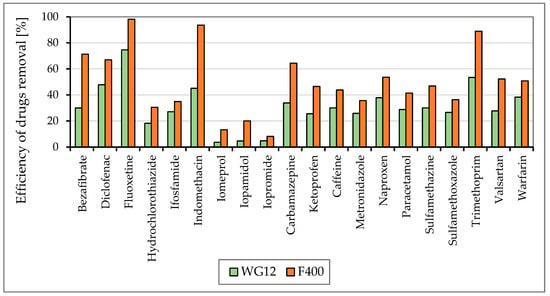
Figure 5.
The efficiency of drug removal (in %) before and after 60 min adsorption with WG12 and F400 at the pH value 7.8.
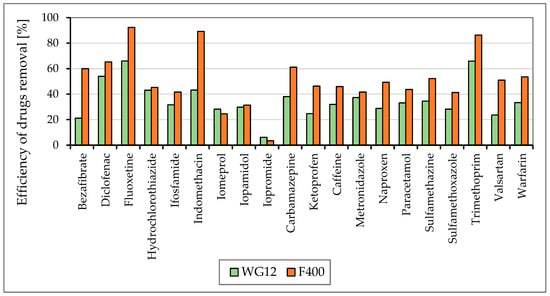
Figure 6.
The efficiency of drug removal (in %) before and after 60 min adsorption with WG12 and F400 at the pH value 6.5.
As is obvious from Figure 3 and Figure 4 the sorption materials F400 and WG12 have different adsorption efficiency for all pharmaceuticals used in this study. Adsorption efficiency ranges from 4 to 98%. There are few differences between pH value of water, but they are very significant. The pH value affects the efficiency of the removal of individual drugs.
Adsorption capacity for adsorption materials used was different. Adsorption capacity for F400 and contact time 60 min was between 0.023 to 214 µg/g, and for WG12, it ranged from 0.006 to 0.163 µg/g.
4. Conclusions
The results shows that Filtrasorb F400 achieved a higher drug removal efficiency from water than the WG12 material. At the same time, the results show the different effectiveness for individual drug. Therefore, it is necessary to verify the use of granular activated carbon directly for a specific contaminant and the quality of treated water. For some drugs, the pH of the water and the contact time of the water with granular activated carbon also play an important role. The higher the contact time, the greater the resulting effect of the method used. After 4 h of sorption, the removal efficiency of contaminants from water was more than 90% for each material.
Author Contributions
J.I. and D.B. worked out a concept and plan of experiments, ensured the installation of all equipment, assembly and verification of used technologies. D.B. and J.I. performed all experiments and water sampling. J.I. analyzed water samples (pharmaceuticals were analyzed in ALS laboratory of Prague, Czech Republic). D.B. and J.I. evaluated the obtained results from experiments. All authors have read and agreed to the published version of the manuscript.
Funding
The experiments were financially supported by Slovak Research and Development Agency of the Slovak Republic (Projects APVV-18-0205 and APVV-22-0610) and by the Ministry of Education, Science, Research and Sports of the Slovak Republic (Project VEGA 1/0825/21).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be available on request.
Acknowledgments
Thanks go to the workers at the ALS laboratory Prague, Czech Republic.
Conflicts of Interest
The authors declare no competing interests.
References
- Rosenfeld, P.; Feng, L. Risk of Hazardous Wastes, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2011; p. 472. [Google Scholar]
- Yang, Y.; Ok, Y.S.; Kim, K.-H.; Kwon, E.E.; Tsang, Y.F. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review. Sci. Total Environ. 2017, 596–597, 303–320. [Google Scholar] [CrossRef] [PubMed]
- Fent, K.; Weston, A.A.; Caminada, D. Ecotoxicology of human pharmaceuticals. Aquat. Toxicol. 2006, 76, 122–159. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Communication from the Commission to the European Parliament, the Council and the European Economic and Social Committee: European Union Strategic Approach to Pharmaceuticals in the Environment. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:52019DC0128&from=SK (accessed on 2 July 2023).
- WHO. Pharmaceuticals in Drinking-Water; WHO Press: Geneva, Switzerland, 2012. [Google Scholar]
- Aus der Beek, T.; Weber, F.-A.; Bergmann, A.; Hickmann, S.; Ebert, I.; Hein, A.; Küster, A. Pharmaceuticals in the environment: Global occurrences and perspectives. Environ. Toxicol. Chem. 2016, 35, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Mackuľak, T.; Czölderová, M.; Grabic, R.; Bodík, I.; Vojs-Staňová, A.; Žabka, D.; Horáková, I. Výskyt liečiv a drog v povrchových vodách Slovenska. In Proceedings of the 38th International Scientific Symposium, Svit, Slovakia, 13–15 June 2018; pp. 91–105. [Google Scholar]
- Baresel, C.; Ek, M.; Ejhed, H.; Allard, A.S.; Magnér, J.; Dahlgren, L.; Westling, K.; Wahlberg, C.; Fortkamp, U.; Söhr, S.; et al. Sustainable treatment systems for removal of pharmaceutical residues and other priority persistent substances. Water Sci. Technol. 2019, 79, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bai, Z. Fe-based catalysts for heterogeneous catalytic ozonation of emerging contaminants in water and wastewater. Chem. Eng. J. 2017, 312, 79–98. [Google Scholar] [CrossRef]
- Cuerda-Correa, E.M.; Alexandre-Franco, M.F.; Fernández-González, C. Advanced Oxidation Processes for the Removal of Antibiotics from Water. An Overview. Water 2020, 12, 102. [Google Scholar] [CrossRef]
- Vieno, N.; Tuhkanen, T.; Kronberg, L. Removal of Pharmaceuticals in Drinking Water Treatment: Effect of Chemical Coagulation. Environ. Technol. 2006, 27, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Kabelíková, E. Sledování Účinnosti Vybraných Adsorbentů na Odstraňování Mikropolutantů z Pitné Vody. Diploma Thesis, VUT Brno, Brno, Czech Republic, 2019. [Google Scholar]
- Moravčíková, S. Sledování Účinnosti Odstraňování Léčiva z Vody Vybranými Adsorbenty. Diploma Thesis, VUT Brno, Brno, Czech Republic, 2020. [Google Scholar]
- Vieno, N.M.; Härkki, H.; Tuhkanen, T.; Kronberg, L. Occurrence of pharmaceuticals in river water and their elimination in a pilot-scale drinking water treatment plant. Environ. Sci. Technol. 2007, 41, 5077–5084. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.W.; Choi, D.J.; Kim, S.K.; Her, N.; Zoh, K.D. Adsorption characteristics of selected hydrophilic and hydrophobic micropollutants in water using activated carbon. J. Hazard. Mater. 2014, 270, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Katsigiannis, A.; Noutsopoulos, C.; Mantziaras, J.; Gioldasi, M. Removal of emerging pollutants through Granular Activated Carbon. Chem. Eng. J. 2015, 280, 49–57. [Google Scholar] [CrossRef]
- Licona, K.P.M.; Geaquinto, L.R.d.O.; Nicolini, J.V.; Figueiredo, N.G.; Chiapetta, S.C.; Habert, A.C.; Yokoyama, L. Assessing potential of nanofiltration and reverse osmosis for removal of toxic pharmaceuticals from water. J. Water Process Eng. 2018, 25, 195–204. [Google Scholar] [CrossRef]
- Lu, Z.-Y.; Ma, Y.-L.; Zhang, J.-T.; Fan, N.-S.; Huang, B.-C.; Jin, R.-C. A critical review of antibiotic removal strategies: Performance and mechanisms. J. Water Process Eng. 2020, 38, 101681. [Google Scholar] [CrossRef]
- Homem, V.; Santos, L. Degradation and removal methods of antibiotics from aqueous matrices—A review. J. Environ. Manag. 2011, 92, 2304–2347. [Google Scholar] [CrossRef] [PubMed]
- Pertile, E.; Dvorský, T.; Václavík, V.; Heviánková, S. Use of Different Types of Biosorbents to Remove Cr (VI) from Aqueous Solution. Life 2021, 11, 240. [Google Scholar] [CrossRef] [PubMed]
- Pertile, E.; Václavík, V.; Dvorský, T.; Heviánková, S. The Removal of Residual Concentration of Hazardous Metals in Wastewater from a Neutralization Station Using Biosorbent—A Case Study Company Gutra, Czech Republic. Int. J. Environ. Res. Public Health 2020, 17, 7225. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yin, R.; Zeng, L.; Zhu, M. A review of graphene-based nanomaterials for removal of antibiotics from aqueous environments. Environ. Pollut. 2019, 253, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Li, X.; Xing, J.; Xu, G. Adsorption of potentially toxic elements in water by modified biochar: A review. J. Environ. Chem. Eng. 2020, 8, 104196. [Google Scholar] [CrossRef]
- Kim, S.; Chu, K.-H.; Al-Hamadani, Y.A.J.; Park, C.-M.; Jang, M.; Kim, D.-H.; Yu, M.; Heo, J.; Yoon, Y. Removal of contaminants of emerging concern by membranes in water and wastewater: A review. Chem. Eng. J. 2018, 335, 896–914. [Google Scholar] [CrossRef]
- Ilavský, J.; Barloková, D.; Marton, M. Removal of Specific Pharmaceuticals from Water using Activated Carbon. IOP Conf. Ser. Earth Environ. Sci. 2021, 906, 012065. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).