Abstract
Acidic mine drainage (AMD) discharged from the abandoned Smolník mine (Pech shaft, Slovakia) contaminates surface water in the Smolník creek due to the decreasing pH and the production of heavy metals. Mixing AMD with surface waters results in an increase in pH, which affects the metal precipitation. Using statistical methods, the effect of pH on the concentration of selected metals (Fe, Mn, Al, Cu and Zn) in the water of the contaminated Smolník creek is described in this work. Polynomial curves were used to identify trends in pH and metal concentration in the surface water. The analysis showed that the second-degree polynomial functions as a candidate for explaining metals’ concentration based on the measured surface water’s pH with a goodness of model fit, based on a coefficient of determination ranging from 0.4 to 0.7 depending on the determined metal concentration and location.
1. Introduction
A specific source of environmental contamination is acid mine drainage (AMD). Its production represents one of the biggest environmental problems already during mining, but especially after mining in polymetallic deposits containing sulfides. When exposed to water and oxygen, most sulfide minerals can oxidize, producing sulfuric acid, metal sulfates that can contaminate surface and groundwater [1]. The source of AMD is primarily the remains of mining activities, e.g., flooded shafts and tunnels, heaps and tailings ponds, representing so-called old mining loads [2,3].
Mining waters are formed during mining, but especially after the end of mining of mineral raw materials in the contact zones of the water and geological environment [4,5,6]. The amount and composition of minerals in the deposit have a significant influence on the pH of mine water [7,8]. Acid mine drainage (AMD) with pH values below 4.5 occur mainly in sulphide deposits. Their formation is also influenced by iron and the sulfur oxidizing bacteria of the genus Acidithiobacillus. They are autochthonous microorganisms that occur in ore and coal mines where pyrite is found [9]. Metal cations dissolved in AMD are transported to surface waters. Dissolved Fe2+ ions are oxidized on the surface by oxygen from the air to Fe3+. This chemical reaction is accompanied by the formation of ocher precipitates (e.g., goethite, jarosite and schwertmanite), which absorb some of the metals on their surface [10]. Heavy metals and sulfates present in AMD contaminate groundwater and surface water, which has a negative impact not only on aquatic organisms, but also on soil contamination and the food chain [11].
There are several mines with AMD generation in the Slovak Republic. The Smolník deposit is one of the historically best-known and richest Cu-Fe ore deposits in Slovakia. After the end of mining in 1990, the mine was flooded, and in 1994, AMD penetrated the surface water, which had a negative impact on aquatic organisms. Because it is a partially open geochemical system into which rainwater and surface water flow, the formation of AMD in the Smolník area cannot be stopped and there is no chance of improving the situation [12,13]. Therefore, the abandoned mining area of Smolník in Slovakia currently belongs to the old environmental burden. The oxidation of pyrite and formation of free sulfuric acid causes water acidification and the dissolution of heavy metals from metal ores. This AMD acidifies and contaminates the waters of the Smolník creek, which transfers pollution to the Hnilec basin [12,14,15,16]. Increasing the pH of water is associated with the precipitation of metals in the form of hydroxides. Precipitated metals are subsequently accumulated in sediments and can be released again into the aquatic environment upon changes in hydrobiological and physicochemical conditions, such as pH, redox potential, and salinity [11,17,18,19]. The influence of physicochemical conditions on the accumulation of metals in bottom sediments is also the subject of many investigations [20,21,22,23,24,25]. They are mainly aimed at studying the influence of pH, redox potential or salinity on the behavior of metals in the water environment.
Polynomial curve fitting is a valuable statistical technique employed in the analysis of chemical results. This equation can help to model the relationship between variables, estimate unknown values, and identify trends or patterns within the chemical data [26].
The aim of this study is to use statistical methods to analyze the effect of acidic mine drainage from the Pech shaft (Smolník mine) on the quality of surface water in the Smolník creek, as well as the effect of pH on the concentration of selected metals in the water.
2. Materials and Methods
Two sampling sites along the Smolník creek were selected for the study of surface water quality (1—approx. 200 m from the Pech shaft, 2—tributary to Hnilec (approx. 9 km)). AMD quality from the Pech shaft was also monitored. The samples were taken in the years 2006–2021. Surface water samples were filtered into a 100 mL plastic container and acidified with 2 mL of ultrapure HNO3 (67%).
The physical and chemical parameters of the water were determined by a METTLER TOLEDO multifunctional device in situ and the chemical analysis of the water by the AAS method (SpectrAA-30, Varian, Australia).
The results of the measurements and analyses were further evaluated by statistical methods. For the determination of the dependency between concentrations of individual metals and the resulting water pH we have used polynomial fitting. Multiple degrees of polynomials were tested with the coefficient of determination (r2) being the main criterion for curve selection. Mean squared error (MSE) was used as a secondary, supportive, polynomial selection indicator.
3. Results and Discussion
The average values of metal concentrations and pH values of the surface water (sampling points 1 and 2 from the Smolník creek and acidic mine water from the Pech shaft) during the years 2006–2021 are shown in Table 1. The results are compared with the limit values according to Regulation of the Government of the Slovak Republic no. 269/2010 Coll. Table 1 shows that AMD discharge from the Pech shaft has a permanent negative effect on the water quality in the Smolník creek (samples 1 and 2). Of the monitored metals, the concentrations of elements Fe, Mn, Al, Cu and Zn exceed the limit values. The increase in the concentration of metals is accompanied by a decrease in the pH of the surface water. At the same time, chemical analysis showed that all monitored parameters are exceeded in the AMD.

Table 1.
The results of chemical analysis of water in 2006–2021—Smolník creek and Pech shaft.
As can be seen from Table 1, the concentrations of iron, manganese, aluminum, copper and zinc were exceeded in the Smolník creek. It is known from the literature and our previous studies [11,13,18,20,24] that the behavior of metals in aqueous solutions is influenced by pH, which affects their precipitation and deposition in sediments. Knowledge about the precipitation intervals of selected metals was used to study the effect of pH on the concentration of Al, Cu, Zn, Mn, Fe in the surface water of the Smolník creek.
The statistical analysis was performed for the metal concentrations in water samples taken at sampling points 1 and 2 in the Smolník creek (Table 1). In Figure 1, Figure 2, Figure 3, Figure 4 and Figure 5 are graphically shown dependences of the concentration of the evaluated metals depending on the pH of the surface water.

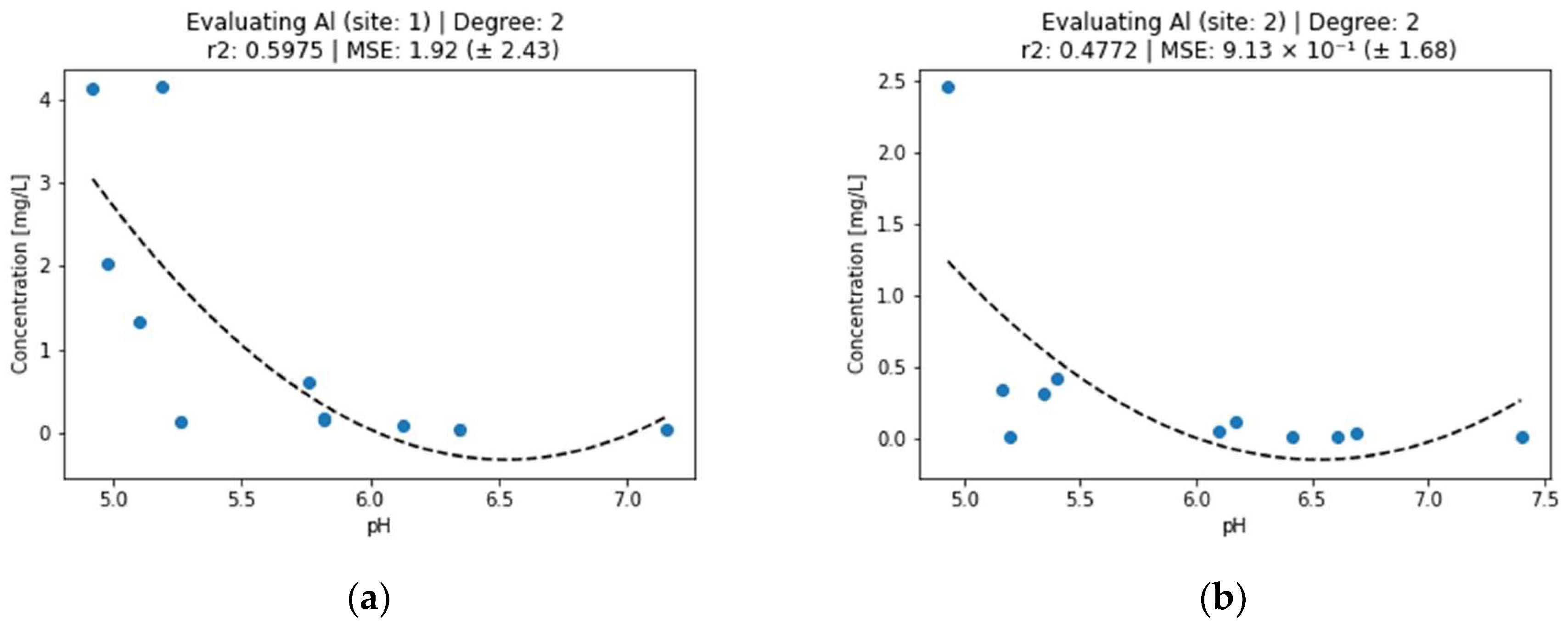
Figure 1.
Effect of pH on aluminum concentration in surface water (a) sampling point 1, (b) sampling point 2.

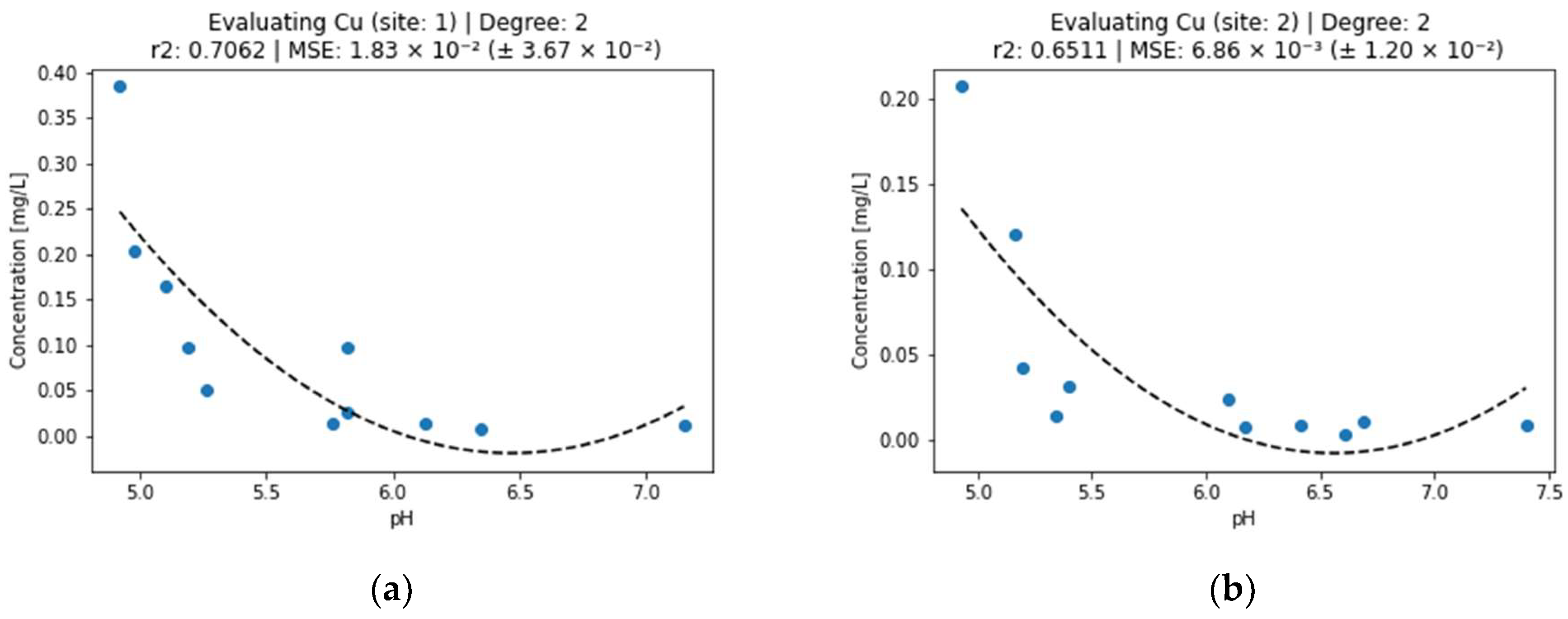
Figure 2.
Effect of pH on copper concentration in surface water (a) sampling point 1, (b) sampling point 2.

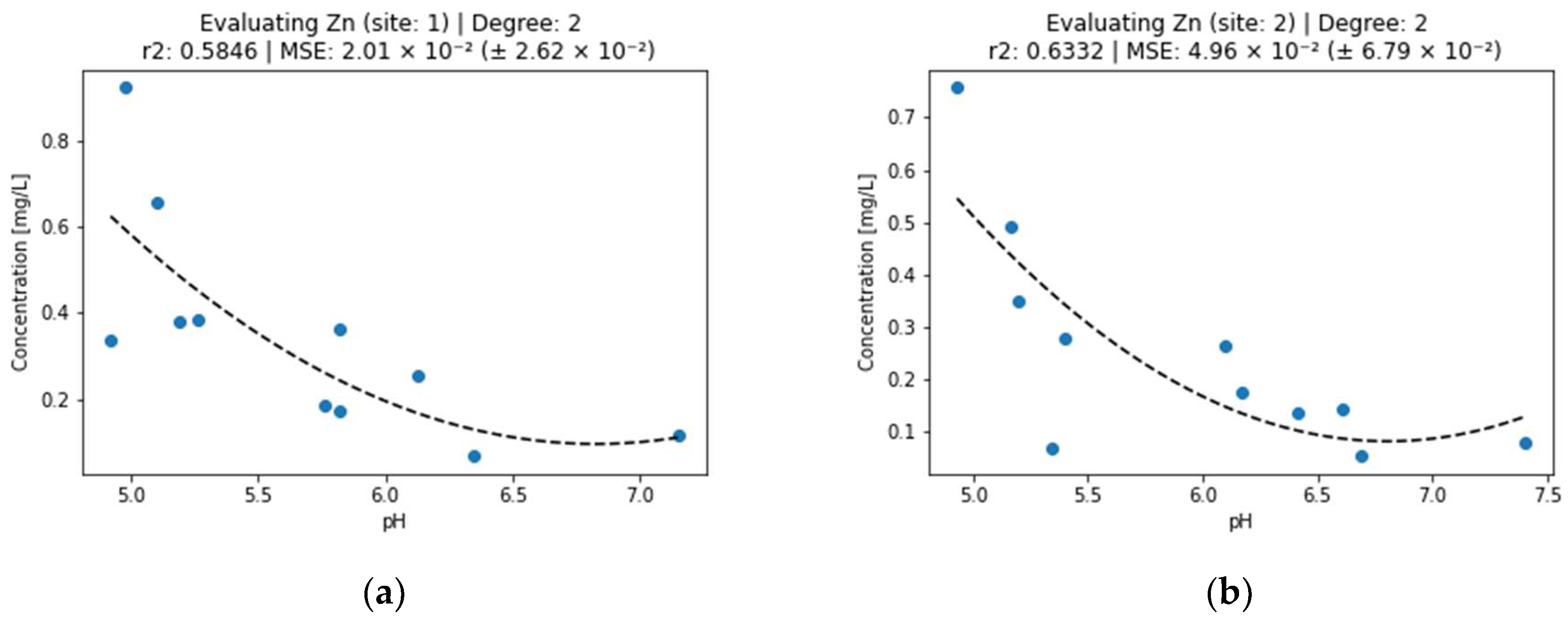
Figure 3.
Effect of pH on zinc concentration in surface water (a) sampling point 1, (b) sampling point 2.

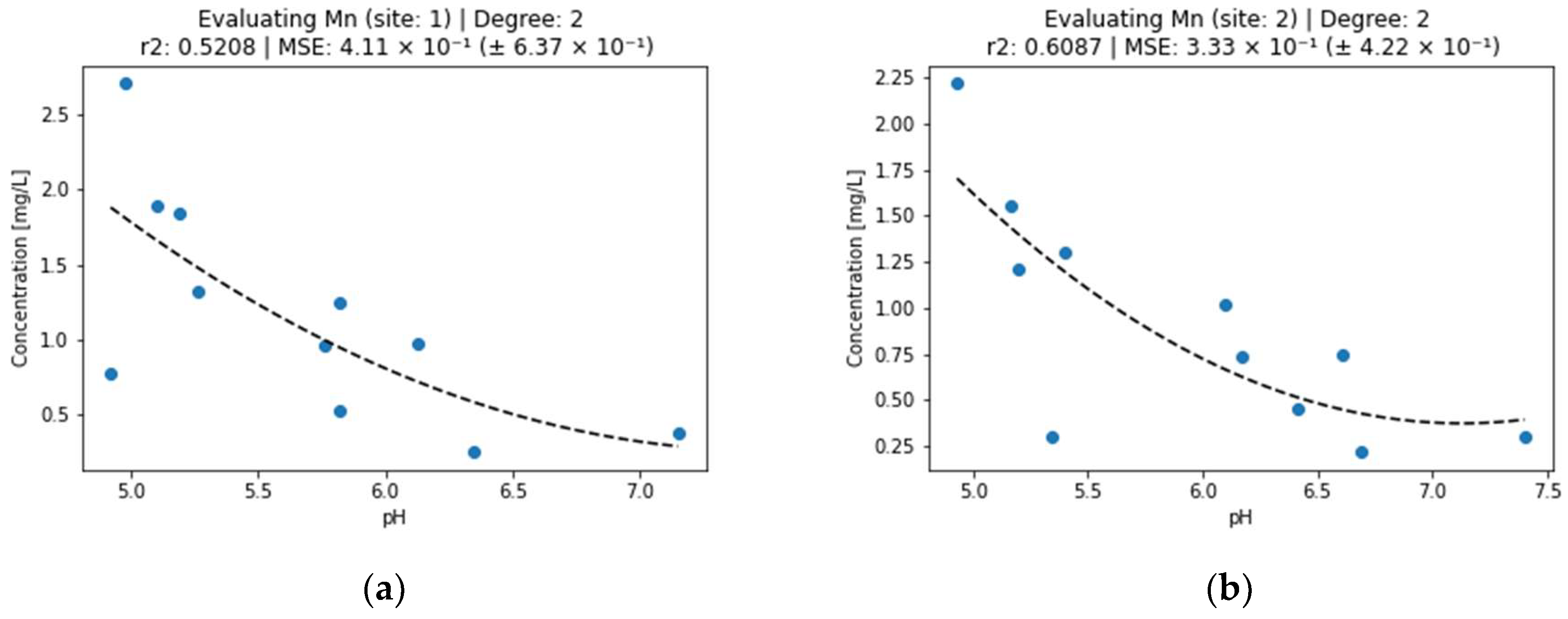
Figure 4.
Effect of pH on manganese concentration in surface water (a) sampling point 1, (b) sampling point 2.

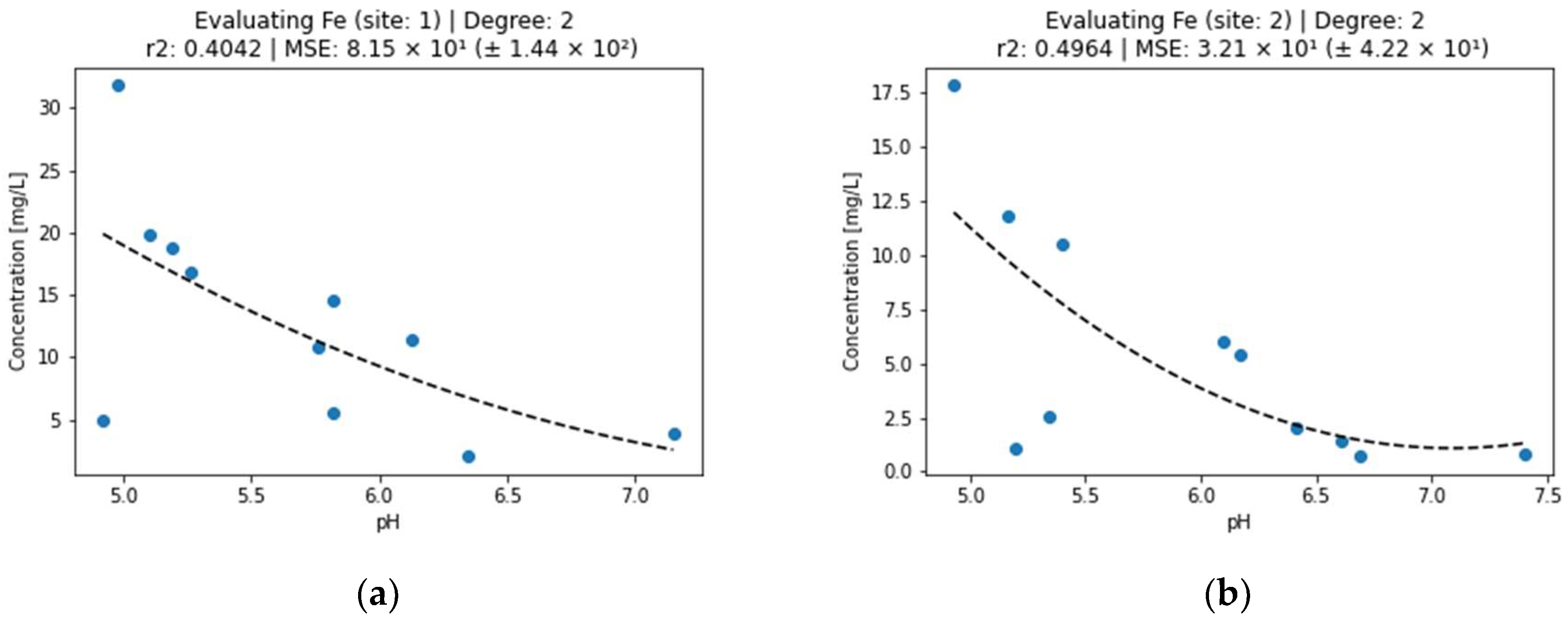
Figure 5.
Effect of pH on iron concentration in surface water (a) sampling point 1, (b) sampling point 2.
In order to statistically evaluate all measurements from both measurement sites for multiple degrees of polynomials, we fitted the respective polynomial curves with a scikit-learn toolbox library available for the programming language Python. The computational evaluation allowed us to test in multiple iterations the polynomial curves of multiple degrees, originally from degree 2 to 10. As a threshold for the best curve candidates’ selection, r2 was set to a minimum of 0.5; only for Al and Fe did we need to lower the threshold to 0.45 in the case of Al and to 0.40 for Fe. This was eliminated for all studied chemical elements polynomials of a degree higher than 3. Afterwards, we evaluated r2 in combination with a mean squared error (MSE) to fine-tune the obtained results. The best fitted curves, all with a second-degree polynomial function, are characterized in Figure 1, Figure 2, Figure 3, Figure 4 and Figure 5.
Figure 1 shows that aluminum concentration decreases with increasing pH. This is in agreement with the literature where aluminum hydroxide precipitates at pH > 5.0 but redissolves at pH > 9.0 [27,28]. The decrease in Al concentration in Figure 1b is also related to the distance of the sampling point from the source of contamination and the longer time of interaction of the contaminant with the water. Looking at the second-degree polynomial curves for Al, we can observe that pH explains the concentration better for site 1—with r2 (0.5975) being higher than for the second site (0.4772).
The precipitation of copper and thus the reduction in its concentration in the water (Figure 2) was carried out in accordance with the literature, according to which copper precipitates at pH > 4 and completely precipitates at pH 6 [19,20]. As can be seen in Figure 2, the concentration of Cu at pH 6 was lower than 0.05 mg/L. Concentrations of copper in both sites have the best explainability by pH from all analyzed metals. Similarly to aluminum, site 1 has a stronger relation between pH and concentration than site 2.
According to [19,27], zinc precipitates in the pH range of 5.5–7. This was reflected in a decrease in concentration below 0.1 mg/L at a pH above 6 (Figure 3). Zinc has the second best explainability of a relationship between water pH and the concentration of the metal, right after copper. In this case, site 2 concentrations are better explained by pH (r2 = 0.6332) than the concentrations of the first site (r2 = 0.5846) by using second-degree polynomials.
Manganese is a common pollutant in mine waters worldwide [29]. Although the hydroxide precipitation of metal cations is usually an effective method for their elimination from aqueous solutions (eg Fe, Cu, Zn, Ni), it is not effective in reducing Mn concentrations below 1 mg/L. In the work [30], various procedures for removing Mn from wastewater from different sources and compositions are presented, while confirming the highest efficiency at pH 8.5 and higher. This statement is not completely valid for Mn in surface waters. Figure 4 shows the decrease in Mn concentration below 0.5 mg/L at pH around 7. Similarly to zinc, the manganese concentrations can also be better explained by the pH for the second site. In this case, the r2 coefficient is between 0.5 and 0.6.
Iron should occur in AMD mainly as an Fe2+ cation, which precipitates at pH < 8.5 [27]. The fact that iron precipitated throughout the studied interval was caused by the oxidation of Fe2+ to Fe3+ by atmospheric oxygen and the precipitation of Fe(OH)3, which starts at pH 3.5. The values of the determination coefficients r2 < 0.5 for the dependence of Fe concentration on pH (Figure 5) confirm a different course of Fe precipitation due to the simultaneous oxidation of Fe2+ to Fe3+ [18,20]. This simultaneous oxidation explains the worst goodness of fit for the second-degree polynomial curves we use in this study. The relationship was the weakest of all studied metals.
4. Conclusions
The location of the Smolník mine is included among the old environmental burdens due to AMD production and surface water contamination in the Smolník creek. This fact was confirmed by exceeding the limit values of pH and the monitored concentrations of heavy metals in the surface water according to Slovak legislation. Fluctuations in the pH value also affect the concentration of heavy metals (e.g., Fe, Cu, Zn, Al, Mn) in the Smolník creek polluted by acid mine drainage, which was confirmed by the presented results. Statistical analysis showed that pH has a significant effect on the concentration of metals in surface water. It is important to note that this relationship cannot be properly interpreted without a deeper understanding of the chemical properties of the studied metals. The weakest link observed in iron can be explained by the simultaneous oxidation of Fe2+ to Fe3+. On the other hand, the best explainability of metal concentration by pH was achieved for copper, using a second-degree polynomial.
Author Contributions
Conceptualization, M.B. and N.J.; methodology, M.B.; software, Y.C.; validation, M.B., N.J. and Y.C.; formal analysis, M.B.; investigation, N.J.; data curation, M.B.; writing M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Scientific Grant Agency of the Ministry of Education, Science, Research and Sport of the Slovak Republic and the Slovak Academy of Sciences, project VEGA Grant No. 2/0108/23 and APVV-20-0140.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- Akcil, A.; Koldas, K. Acid mine drainage (AMD): Causes, treatment and case studies. J. Clean. Prod. 2006, 14, 1139–1146. [Google Scholar] [CrossRef]
- Lintnerová, O.; Šottník, P.; Šoltés, S. Abandoned Smolnik mine (Slovakia) a catchment area affected by mining activities. Estonian J. Earth Sci. 2008, 57, 104–110. [Google Scholar] [CrossRef]
- Dvořáček, J.; Malíková, P.; Sousedíková, R.; Heviánková, S.; Rys, P.; Osičková, I. Water production as an option for utilizing closed underground mines. J. S. Afr. Inst. Min. Metall. 2022, 122, 571–577. [Google Scholar]
- Skousen, J.G.; Ziemkiewicz, P.F.; McDonald, L.M. Acid mine drainage formation, control and treatment: Approaches and strategies. Extr. Ind. Soc. 2019, 6, 241–249. [Google Scholar] [CrossRef]
- Yuan, J.; Ding, Z.; Bi, Y.; Li, J.; Wen, S.; Bai, S. Resource Utilization of Acid Mine Drainage (AMD): A Review. Water 2022, 14, 2385. [Google Scholar] [CrossRef]
- Nishimoto, N.; Yamamoto, Y.; Yamagata, S.; Igarashi, T.; Tomiyama, S. Acid Mine Drainage Sources and Impact on Groundwater at the Osarizawa Mine, Japan. Minerals 2021, 11, 998. [Google Scholar] [CrossRef]
- Valová, B.; Kotalová, I.; Heviánková, S. Determination of Risk Elements in Mine Waste Dump Soil Sample Using Sequential BCR Extraction. Inzynieria Miner. 2020, 2020, 217–220. [Google Scholar]
- Archundia, D.; Prado-Pano, B.; González-Méndez, B.; Loredo-Portales, R.; Molina-Freaner, F. Water resources affected by potentially toxic elements in an area under current and historical mining in northwestern Mexico. Environ. Monit. Assess. 2021, 193, 236. [Google Scholar] [CrossRef]
- Johnson, D.B.; Hallberg, K.B. Acid mine drainage remediation options: A review. Sci. Total Environ. 2005, 338, 3–14. [Google Scholar] [CrossRef]
- Andras, P.; Adam, M.; Chovan, M.; Slesarova, A. Environmental hazards of the bacterial leaching of ore minerals from waste at the Pezinok deposit (Malé Karpaty MTS., Slovakia). Carpathian J. Earth Environ. Sci. 2008, 3, 7–22. [Google Scholar]
- Hakansson, K.; Karlsson, S.; Allard, B. Effects of pH on the accumulation and redistribution of metals in polluted stream bed sediment. Sci. Total Environ. 1989, 87/88, 43–57. [Google Scholar] [CrossRef]
- Luptakova, A.; Kusnierova, M. Bioremediation of Acid Mine Drainage by SRB. Hydrometallurgy 2005, 77, 97–102. [Google Scholar] [CrossRef]
- Singovszka, E.; Balintova, M.; Holub, M. Heavy metal contamination and its indexing approach for sediment in Smolnik creek (Slovakia). Clean Technol. Environ. Policy 2016, 18, 305–313. [Google Scholar] [CrossRef]
- Bajtoš, P. Mine waters in the Slovak part of the Western Carpathians: Distribution, classification and related environmental issues. Slovak Geol. Mag. 2016, 16, 139–158. [Google Scholar]
- Kupka, D.; Pállová, Z.; Horňáková, A.; Achimovičová, M.; Kavečanský, V. Effluent water quality and the ochre deposit characteristics of the abandoned Smolnik mine, East Slovakia. Acta Montan. Slovaca 2012, 17, 56–64. [Google Scholar]
- Lintnerová, O.; Šottník, P.; Šoltés, S. Stream sediment and soil pollution in the Smolnik mining area (Slovakia). Slovak Geol. Mag. 2003, 9, 201–203. [Google Scholar]
- Calmano, W.; Hong, J.; Forstner, U. Binding and mobilization of heavy metals in contaminated sediments affected by pH and redox potential. Water Sci. Technol. 1993, 28, 223–235. [Google Scholar] [CrossRef]
- Balintova, M.; Singovszka, E.; Vodicka, R.; Purcz, P. Statistical Evaluation of Dependence Between pH, Metal Contaminants, and Flow Rate in the AMD-Affected Smolnik Creek. Mine Water Environ. 2016, 35, 10–17. [Google Scholar] [CrossRef]
- Kruopiene, J. Distribution of Heavy Metals in Sediments of the Nemunas River (Lithuania). Polish J. Environ. Stud. 2007, 16, 715–722. [Google Scholar]
- Balintova, M.; Petrilakova, A. Study of pH Influence on Selective Precipitation of Heavy Metals from Acid Mine Drainage. Chem. Eng. Trans. 2011, 25, 345–350. [Google Scholar]
- Funes, A.; De Vicente, J.; Cruz-Pizarro, L.; De Vicente, I. The influence of pH on manganese removal by magnetic microparticles in solution. Water Res. 2014, 53, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Aldrich, C.; Tan, H. Treatment of acid mine water by use of heavy metal precipitation and ion exchange. Miner. Eng. 2000, 13, 623–642. [Google Scholar] [CrossRef]
- Balintova, M.; Petrilakova, A.; Singovszka, E. Study of metal ion sorption from acidic solutions. Theor. Found. Chem. Eng. 2012, 46, 727–731. [Google Scholar] [CrossRef]
- Balintova, M.; Petrilakova, A.; Singovszka, E. Study of metals distribution between water and sediment in the Smolnik Creek (Slovakia) contaminated by acid mine drainage. Chem. Eng. Trans. 2012, 28, 73–78. [Google Scholar]
- Luptakova, A.; Balintova, M.; Jencarova, J.; Macingova, E.; Prascakova, M. Metals recovery from acid mine damage. Nova Biotechnol. 2010, 22, 1111–1118. [Google Scholar]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Xinchao, W.; Roger, C.; Viadero, J.; Karen, M. Recovery of Iron and Aluminum from Acid Mine Drainage by Selective Precipitation. Environ. Eng. Sci. 2005, 22, 745–755. [Google Scholar]
- Jenke, D.R.; Diebold, F.E. Recovery of valuable metals from acid mine drainage by selective titration. Waters Res. 1983, 17, 1585–1590. [Google Scholar] [CrossRef]
- Kulkarni, S.J. A Review on Studies and Research on Manganese Removal. Int. J. Sci. Res. 2016, 1, 45–48. [Google Scholar]
- Patil, D.S.; Chavan, S.M.; Oubagaranadin, J.U.K. A review of technologies for manganese removal from wastewaters. J. Environ. Chem. Eng. 2016, 4, 468–487. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).