Abstract
The emergence of new biotechnologies has increased interest in the study of the impact of environmental contamination on microbial communities, particularly in relation to the potential benefits that stress-adapted microorganisms offer in environmental protection, industrial ecology, and mineral and waste processing. This study aimed to compare the bioaccumulation abilities of recently isolated microscopic fungi belonging to the genera Aspergillus, Phoma, Cystobasidium, Cladosporium and Exophiala. These fungi were isolated from a site contaminated with both toxic metals and organic pollutants. The study monitored the bioaccumulation of selected toxic metal ions (Cu(II), Zn(II), Ni(II), Cr(III), Pb(II)), as well as pH changes, over a 30-day of biomass growth. The medium containing Pb(II) exhibited a statistically significant pH change during the 30-day accumulation period (Mann-Whitney U Test, p < 0.05). These findings provide valuable insights for the potential industrial application of microscopic fungi in bioaccumulation processes.
1. Introduction
Fungi have garnered significant scientific attention owing to their capacity for biotransformations of toxic metals and their adaptability to varying levels of anthropogenic contamination in the surrounding environment [1,2,3,4]. The main sources of metal-resistant species are found in contaminated sites, where indigenous fungal strains isolated from these areas demonstrate remarkable tolerance to elevated heavy metal concentrations [5,6]. The ecological function of fungi is subject to the influence of multiple factors [7], which typically exhibit variability, including soil composition, soil properties, climatic conditions, and the ever-evolving nature of organic and inorganic anthropogenic pollutants due to the introduction of novel chemical compounds and technological advancements. Toxic metals found in soil impact the composition of fungal communities and exert influence on their ecological function, particularly with regards to metabolite production and the subsequent decomposition of organic matter. Changes in fungal community structure may occur as a result of selective pressures exerted on specific species. Research has corroborated the ability of soil-inhabiting fungi to adapt to varying degrees of toxic metal contamination by modifying the abundance and structure of their communities [8]. The relationship between toxic metal contamination and the structure and species composition of fungal microbial communities has been observed in soils contaminated with various toxic metal ions [9,10].
The emergence of novel biotechnological advancements has spurred a growing interest in investigating the effects of toxic metal contamination on microbial communities. This research area is intricately linked to the potential benefits that stress-adapted microorganisms can offer in terms of environmental protection, industrial ecology, and mineral and waste processing sectors. Presently, environments contaminated with multiple pollutants, specifically the co-occurrence of organic matter and toxic metals, pose significant environmental challenges. In such scenarios, the utilization of heterotrophic organisms, including fungi, holds particular promise as they possess the ability to concurrently remove both types of pollutants.
1.1. Characteristics of the Ostramo Lagoons Site
Processes reliant on cellular metabolism, collectively known as bioaccumulation, encompass the transportation of metals across the cell wall and their subsequent localization within specific cellular compartments [11,12]. Numerous microorganisms, including filamentous fungi and yeasts, have demonstrated the capability to accumulate toxic metals within their cellular structures [13,14]. Bioaccumulation is categorized as a physiological mechanism for metal immobilization that is dependent upon cellular metabolism. Unlike biosorption, bioaccumulation is a more intricate process involving active transport of substances across cell membranes facilitated by transport proteins [15,16]. This active transport is preceded by a brief and rapid passive phase occurring immediately upon contact of the organism with toxic metal ions, involving ion exchange at the cell surface and physical adsorption [17].
The success of bioaccumulation relies on the internal structural and biochemical properties of cells, genetic and physiological adaptations of the organism, and the different oxidation states of metals [18]. One limiting factor in the utilization of living biomass for bioremediation processes is the toxicity of the metal to the microorganism. Elevated concentrations of toxicants can damage cell membranes and organelles, induce lipid peroxidation, and disrupt enzymatic functions, leading to the generation of reactive oxygen species and cell apoptosis [19]. Consequently, research efforts should also focus on the potential adaptation of respective organisms to stress conditions, which could enhance their efficiency in metal removal processes [20].
1.2. Effect of pH on Bioaccumulation of Toxic Metals
pH is one of the key factors that influences both the availability of metals and their uptake by fungi [21]. pH determines the chemical form in which a metal exists, whether as an ion, complex compound, or precipitate, which subsequently affects the potential for bioaccumulation by microorganisms [22]. Strains of filamentous fungi tolerant to elevated concentrations of toxic metals may exhibit increased production of organic acids. This phenomenon can be described as one of the resistance mechanisms employed by fungi against toxic metals, as organic acids react with metals to form organic compounds [23,24]. The change in pH in the solution during bioaccumulation may thus be a determining factor indicating the type of resistance mechanism in fungi or may be related to the bioaccumulation potential of the strain. Moreover, the availability and reactivity of fungal cell wall functional groups can be influenced by pH, which is widely used during biosorption processes [25,26].
Furthermore, pH exerts a notable influence on the physiology and metabolism of fungi, leading to indirect effects on metal accumulation. Fungal growth, enzyme activity, and various cellular processes are intricately linked to pH levels. Any pH-induced alterations in these factors can influence the ability of fungi to accumulate and tolerate toxic metals.
2. Materials and Methods
2.1. Characteristics of the Ostramo Lagoons Site
The Ostramo Lagoons, located in Ostrava, Czech Republic, were classified as significant ecological burdens. Their origins could be traced back to the deposition of waste generated from refinery production, which began in the late 19th century. Since 1965, the lagoons also served as a repository for waste resulting from the regeneration of used lubricating oils. The primary organic pollutants found in the lagoons included petroleum hydrocarbons, polycyclic aromatic hydrocarbons, polychlorinated biphenyls, and phenols. In addition to organic pollution, the lagoons were found to contain various toxic metals, including Arsenic, Cadmium, Copper, Mercury, Nickel, and Lead. Furthermore, the pH levels within the lagoons ranged from acidic to neutral [27,28].
2.2. Experimental Bioaccumulation of Toxic Metals by Selected Species of Isolated Microscopic Filamentous Fungi
The present study aimed to investigate the bioaccumulation of toxic metals in the biomass of microscopic fungi under controlled laboratory conditions. The following strains of isolated microscopic fungi were used: Aspergillus niger, Aspergillus candidus, Aspergillus iizukae, Aspergillus westerdijkiae, Aspergillus ochraceus, Aspergillus clavatus, Phoma sp., Cystobasidium sp., Cladosporium sp., Exophiala xenobiotica. The bioaccumulation experiments were conducted using static incubation methods. The respective metal salts solved in demineralized water were initially added to the cultivation medium to achieve a final concentration of 100 mg L−1 of metal. The metal ions investigated included Cu(II) from Cu(NO3)2·3H2O (Sigma Aldrich, Darmstadt, Germany, purity 99.9%), Zn(II) from Zn(NO3)2·6H2O (Sigma Aldrich, purity > 99.0%), Ni(II) from NiCl2·6H2O (Sigma Aldrich, purity > 99.9%), Cr(III) from Cr(NO3)3·9H2O (Sigma Aldrich, purity 99.0%) and Pb(II) from Pb(NO3)2 (Penta Chemicals, Prague, Czech Republic, purity 99.0%).
The cultivation media used were Sabouraud Dextrose Broth (HiMedia Laboratories, Mumbai, India) for Cu(II), Zn(II), Ni(II), and Cr(III), and Czapek Dox Liquid Medium (HiMedia Laboratories) for Pb(II). The preparation of both media followed the instructions provided by the manufacturer, using demineralized water. The resulting pH of Sabouraud Dextrose Broth was measured to be 5.6 ± 0.2, while Czapek Dox Liquid Medium had a pH of 6.8 ± 0.2. Autoclave sterilization was performed at a temperature of 121 °C and a pressure of 15 lbs to ensure sterility of the media.
Static cultivation of the fungi was carried out in Erlenmeyer flasks for a duration of 30 days. The pH of the media was monitored throughout the cultivation period using standardized pH meter (HANNA, HI 991001). After 30 days of growth, the live biomass was harvested, filtered, dried, and weighed. The medium containing metal ions was filtered using a Gridded MCE sterile filter with a pore size of 0.45 μm (Membrane Solutions, Auburn, WA, USA). The metal content in both the biomass and nutrient medium was determined using atomic absorption spectroscopy (AAS) performed on a VARIAN AA 280FS instrument (Agilent, Santa Clara, CA, USA).
The efficiency of bioaccumulation was quantified as the ratio of the amount of metal accumulated by the biomass after 30 days of cultivation to the initial amount of metal present in the nutrient medium prior to the introduction of microscopic fungi according to the following equation:
where µ is expressed as a percentage and represents the efficiency at which a metal is accumulated within the total amount of dried biomass. The parameter macc denotes the amount of metal accumulated, while min represents the initial amount of metal present in the medium at the beginning of the incubation. For instance, in this particular scenario, the metal concentration in the medium is 100 mg L−1 in a 0.5 L volume, resulting in an initial metal amount of 50 mg.
3. Results
The bioaccumulation efficiency shown in Table 1 has been determined by evaluating the obtained data, which quantifies the total amount of toxic metal that has accumulated in the dried biomass, expressed as a percentage according to Equation (1).

Table 1.
Calculation of bioaccumulation efficiency: total amount of toxic metal accumulated in dried biomass (%).
The results regarding bioaccumulation efficiency revealed that A. ochraceus exhibited the highest efficiency among the tested metal ions. It achieved 57.42% efficiency during Cu(II) bioaccumulation, 56.88% efficiency during Zn(II) bioaccumulation, and 37.73% efficiency during Cr(III) bioaccumulation. This strain demonstrates a high tolerance to the toxic effects of these transition metal ions and efficiently accumulates them within its cells, utilizing its metabolic potential.
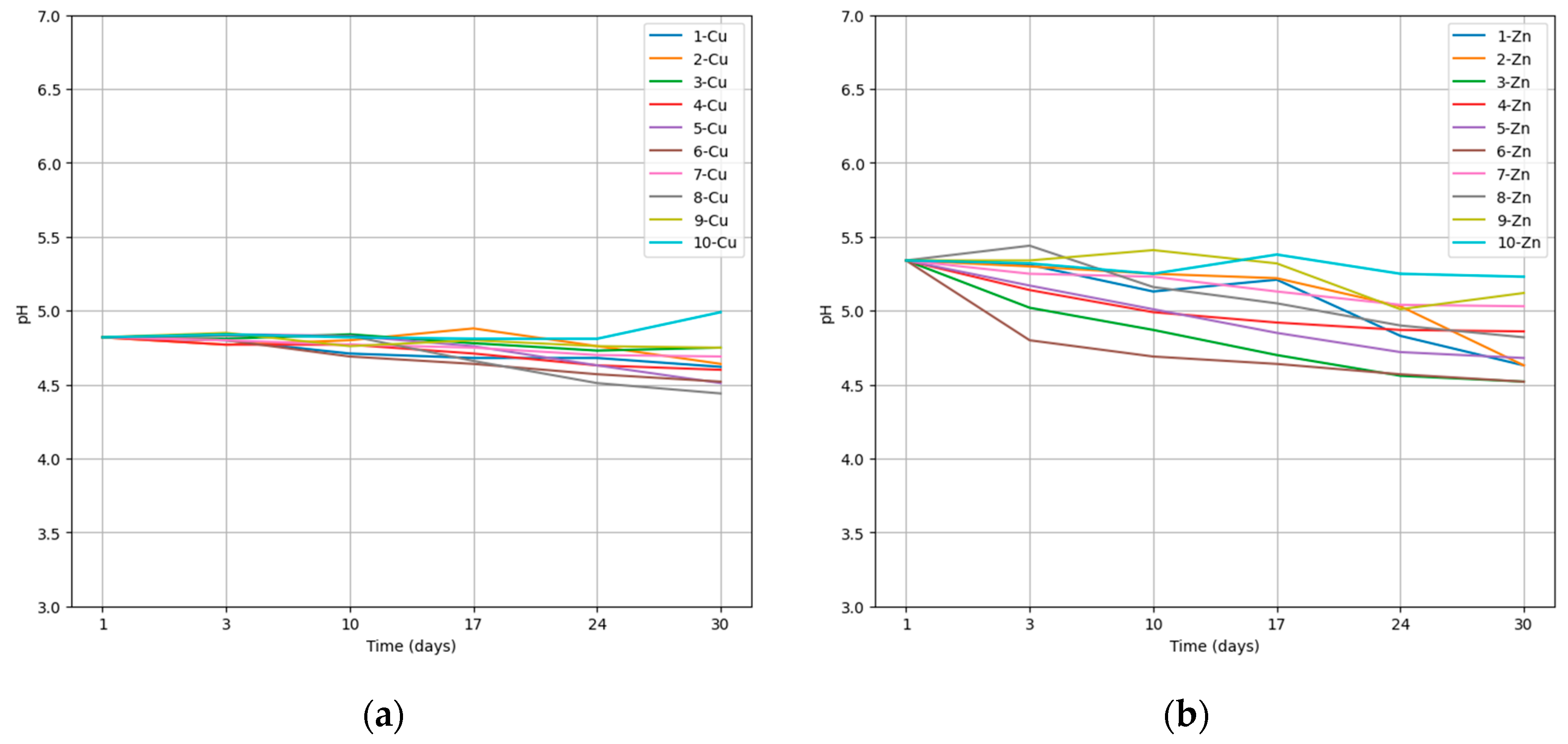
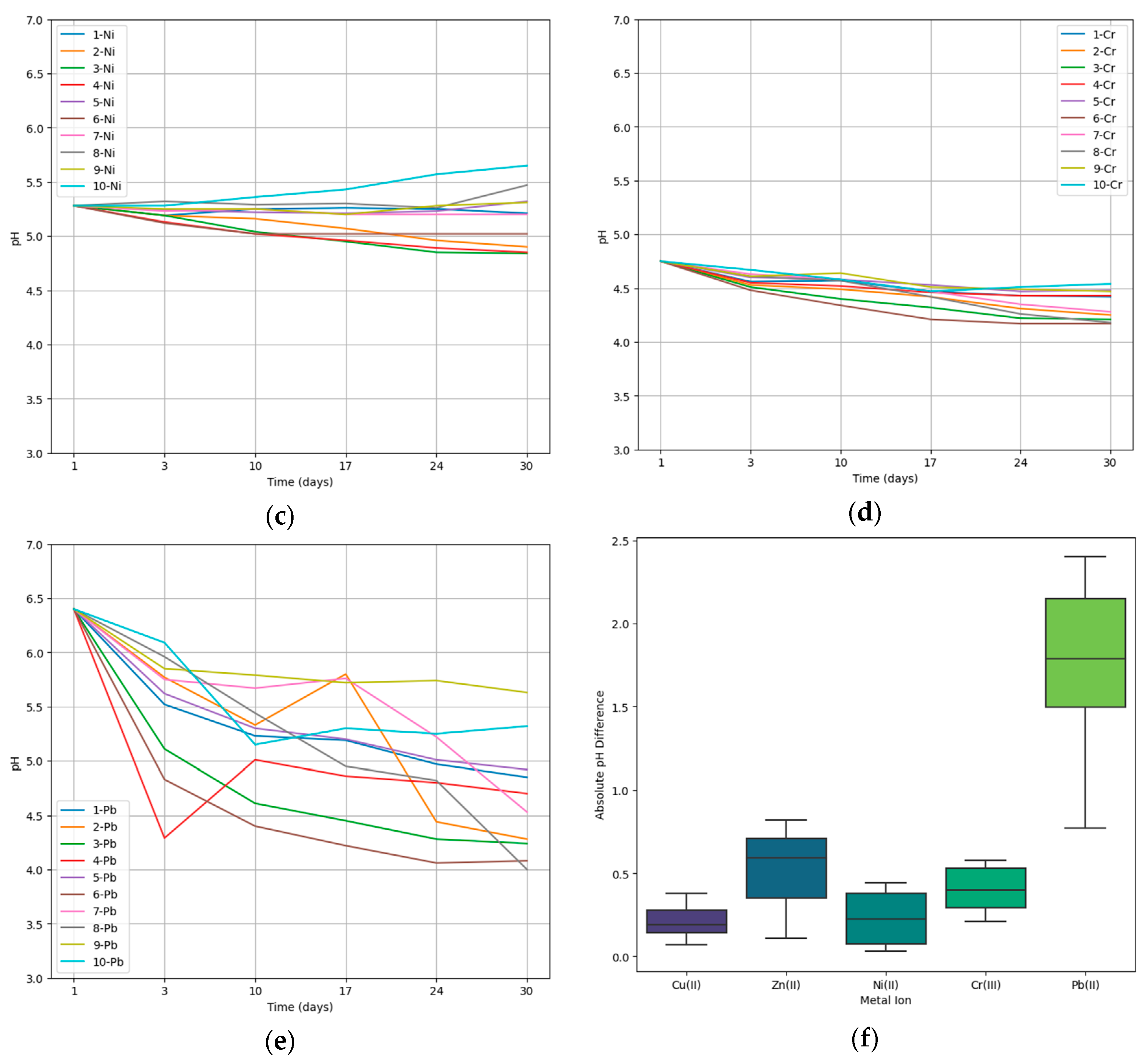
The pH was measured at weekly intervals, starting immediately after the addition of a spore suspension into the medium, and continuing on days 3, 10, 17, 24, and 30. Figure 1 illustrates the pH course in the media containing specific metal ions and the microscopic fungus. The pH values were plotted against time, providing insights into the changing acidity or alkalinity levels. Each graph showcases the pH trends for each specific metal ion and the microscopic fungus under investigation. Additionally, the boxplot in Figure 1f represents the distribution of pH changes in media containing particular metal ions, each plot contains the values of pH change for fungi 1–10.
Figure 1.
The course of pH in the bioaccumulation process. 1—A. niger, 2—A. candidus, 3—A. iizukae, 4—A. westerdijkiae, 5—A. ochraceus, 6—A. clavatus, 7—Phoma sp., 8—Cystobasidium sp., 9—Cladosporium sp., 10—Exophiala xenobiotica (a) Change in pH in Cu-containing media during bioaccumulation; (b) change in pH in Zn-containing media during bioaccumulation.; (c) change in pH in Ni-containing media during bioaccumulation; (d) change in pH in Cr-containing media during bioaccumulation; (e) change in pH in Pb-containing media during bioaccumulation; (f) pH variation in metal ion-enriched media between day 1 and day 30.
4. Discussion
When describing the bioaccumulation of metals present in a nutrient medium through growing biomass, it is appropriate to consider the ways in which growing filamentous microscopic fungi can interact with metals. One possible scenario involves the uptake of metal cations into the cell through membrane transporters and their compartmentalization or biotransformation inside the cell, or conversely, efflux from the cell to the outside [29]. Another mode of interaction involves the production of organic acids, which have the ability to react with metals, resulting in the formation of insoluble compounds that can be present in the nutrient medium and on the surface of fungal mycelia. Certain species of microscopic fungi, such as those belonging to the genus Aspergillus, have the ability to produce extracellular polymeric substances (EPS) that can act as surface-active agents during the removal of toxic metals [30]. These genetically determined mechanisms directly influence the process of metal biotransformation, including the bioaccumulation potential of individual strains.
The average efficiency of bioaccumulation varied depending on the metal being accumulated, with the following preference order: Zn(II) > Cu(II) > Cr(III) > Ni(II) > Pb(II). These findings indicate that fungi possess an enhanced ability to accumulate metal ions, especially those that serve as essential micronutrients like Zn(II) an Cu(II). This heightened accumulation can be attributed to specific mechanisms that facilitate fungi in the efficient uptake and subsequent accumulation of these metals, which play crucial roles in various critical cellular processes [31,32].
To gain further insights into the bioaccumulation process of toxic metals by microscopic fungi, the pH of the enriched medium, which can be influenced by the production of secondary metabolites by the fungi, was measured. Fungi act as chelating agents and detoxify the effects of some toxic metal ions. Metal chelation through oxalic acid form oxalate crystals which can be in form of precipitates in growth medium or can be entrapped in fungal hyphae [24]. Furthermore, a correlation has been demonstrated between the higher production of organic acids by fungi and the degree of adaptation of a specific fungal strain to the presence of toxic metals in the environment [33]. The observed variations in pH within the metal ion-enriched medium underscore the dynamic nature of the bioaccumulation processes. Changes in pH can affect the ionization and speciation of metal ions, thereby affecting their solubility and bioavailability, as well as their ability to bind to specific sites in the fungal cell wall, which is widely exploited during bioremediation [34]. Consequently, these variations influence the interactions between microscopic fungi and the metals [35,36].
The medium containing Pb(II) experienced the largest average change in pH over the 30-day accumulation period. On average, the pH changed by 1.75, which indicates that the presence of Pb(II) had a statistically significant impact on the acidity or alkalinity of the medium (Mann-Whitney U test, p < 0.05). The low average bioaccumulation efficiency for Pb(II) among tested fungal strains (7.01%) is consistent with the observation that low pH values are not effective in removing Pb(II) from aqueous solutions and wastewater [37]. In the case of other metal ions, no significant change was observed. The lowest average change in pH was recorded for Cu(II) and Ni(II). In the case of Ni(II), even in four cases the pH of the medium increased by 0.04, 0.19, 0.03 a 0.37, respectively. Ni(II) was also accumulated with the second lowest average efficiency of the metal ions tested (9.15%), indicating the low ability of tested fungal strains to produce organic acids in the presence of Ni(II) and also to accumulate them inside their cells or in the cell wall.
5. Conclusions
The results of the study highlight that the bioaccumulation potential of microscopic fungi is influenced by both the type of metal ion and the particular strain of autochthonous fungi. The interaction between the growing fungal biomass and the metal ions within the medium encompasses various processes, including bioaccumulation, biosorption, ion exchange, and precipitation, among others. Alterations in the medium’s pH during this process provide insights into the fungus’s environmental interactions. These pH shifts indicate whether the fungus is producing more or fewer organic acids, aiding in the understanding of the underlying biochemical processes within the organism’s resistance mechanisms. Bioaccumulation experiments for 30 days revealed a statistically significant effect of Pb(II) on the resulting pH of the culture medium.
Funding
Research was funded by The Project for Specific University Research (SGS) No. SP2023/4 by the Faculty of Mining and Geology of VŠB—Technical University of Ostrava.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The author declares no conflict of interest.
References
- Farkas, B.; Vojtková, H.; Bujdoš, M.; Kolenčík, M.; Šebesta, M.; Matulová, M.; Duborská, E.; Danko, M.; Kim, H.; Kučová, K.; et al. Fungal mobilization of selenium in the presence of hausmannite and ferric oxyhydroxides. J. Fungi 2021, 7, 810. [Google Scholar] [CrossRef] [PubMed]
- Duborská, E.; Szabó, K.; Bujdoš, M.; Vojtková, H.; Littera, P.; Dobročka, E.; Kim, H.; Urík, M. Assessment of Aspergillus niger strain’s suitability for arsenate-contaminated water treatment and adsorbent recycling via bioextraction in a laboratory-scale experiment. Microorganisms 2020, 8, 1668. [Google Scholar] [CrossRef]
- Urík, M.; Polák, F.; Bujdoš, M.; Miglierini, M.B.; Milová-Žiaková, B.; Farkas, B.; Goneková, Z.; Vojtková, H.; Matúš, P. Antimony leaching from antimony-bearing ferric oxyhydroxides by filamentous fungi and biotransformation of ferric substrate. Sci. Total Environ. 2019, 664, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Šimonovičová, A.; Ferianc, P.; Vojtková, H.; Pangallo, D.; Hanajík, P.; Kraková, L.; Feketeová, Z.; Čerňanský, S.; Okenicová, L.; Žemberyová, M.; et al. Alkaline Technosol contaminated by former mining activity and its culturable autochthonous microbiota. Chemosphere 2017, 171, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Gadd, G.M.; Sayer, J.A. Environmental Microbe-Metal Interactions; Lovley, D.R., Ed.; ASM Press: Washington, DC, USA, 2000; p. 237. [Google Scholar]
- Šimonovičová, A.; Vojtková, H.; Nosalj, S.; Piecková, E.; Švehláková, H.; Kraková, L.; Drahovská, H.; Stalmachová, B.; Kučová, K.; Pangallo, D. Aspergillus niger environmental isolates and their specific diversity through metabolite profiling. Front. Microbiol. 2021, 12, 658010. [Google Scholar] [CrossRef] [PubMed]
- Nosalj, S.; Šimonovičová, A.; Vojtková, H. Enzyme production by soilborne fungal strains of Aspergillus niger isolated from different localities affected by mining. In Proceedings of the IOP Conference Series Earth and Environmental Science, Surakarta, Indonesia, 24–25 August 2021; Volume 900, p. 012027. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, J.; Lu, J.; Sun, Y. Study on the influence of soil microbial community on the long-term heavy metal pollution of different land use types and depth layers in mine. Ecotoxicol. Environ. Saf. 2019, 170, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Rajapaksha, R. Heavy metal tolerance of culturable bacteria and fungi in a long-term cultivated tropical ultisol. Eur. J. Soil Biol. 2011, 47, 9–15. [Google Scholar] [CrossRef]
- Zafar, S.; Aqil, F.; Ahmad, I. Metal tolerance and biosorption potential of filamentous fungi isolated from metal contaminated agricultural soil. Bioresour. Technol. 2007, 98, 2557–2561. [Google Scholar] [CrossRef]
- Blackwell, K.; Tobin, J. Cadmium accumulation and its effects on intracellular ion pools in a brewing strain of Saccharomyces cerevisiae. J. Ind. Microbiol. Biotechnol. 1999, 23, 204–208. [Google Scholar] [CrossRef]
- Gadd, G.M. Biosorption: Critical review of scientific rationale, environmental importance and significance for pollution treatment. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2009, 84, 13–28. [Google Scholar] [CrossRef]
- Šebesta, M.; Vojtková, H.; Cyprichová, V.; Ingle, A.P.; Urík, M.; Kolenčík, M. Mycosynthesis of metal-containing nanoparticles—Fungal metal resistance and mechanisms of synthesis. Int. J. Mol. Sci. 2022, 23, 14084. [Google Scholar] [CrossRef] [PubMed]
- Šebesta, M.; Vojtková, H.; Cyprichová, V.; Ingle, A.P.; Urík, M.; Kolenčík, M. Mycosynthesis of metal-containing nanoparticles—Synthesis by Ascomycetes and Basidiomycetes and their application. Int. J. Mol. Sci. 2023, 24, 304. [Google Scholar] [CrossRef] [PubMed]
- Bibbins-Martínez, M.; Juárez-Hernández, J.; López-Domínguez, J.; Nava-Galicia, S.; Martínez-Tozcano, L.; Juárez-Atonal’, R.; Cortés-Espinosa, D.; Díaz-Godinez, G. Potential application of fungal biosorption and/or bioaccumulation for the bioremediation of wastewater contamination: A review. J. Environ. Biol. 2023, 44, 135–145. [Google Scholar] [CrossRef]
- Argüello, J.M.; Raimunda, D.; González-Guerrero, M. Metal transport across biomembranes: Emerging models for a distinct chemistry. J. Biol. Chem. 2012, 287, 13510–13517. [Google Scholar] [CrossRef] [PubMed]
- Dursun, A.; Uslu, G.; Cuci, Y.; Aksu, Z. Bioaccumulation of copper (II), lead (II) and chromium (VI) by growing Aspergillus niger. Process Biochem. 2003, 38, 1647–1651. [Google Scholar] [CrossRef]
- Timková, I.; Sedláková-Kaduková, J.; Pristaš, P. Biosorption and bioaccumulation abilities of actinomycetes/streptomycetes isolated from metal contaminated sites. Separations 2018, 5, 54. [Google Scholar] [CrossRef]
- Igiri, B.E.; Okoduwa, S.I.; Idoko, G.O.; Akabuogu, E.P.; Adeyi, A.O.; Ejiogu, I.K. Toxicity and bioremediation of heavy metals contaminated ecosystem from tannery wastewater: A review. J. Toxicol. 2018, 2018, 2568038. [Google Scholar] [CrossRef]
- Valix, M.; Loon, L. Adaptive tolerance behaviour of fungi in heavy metals. Miner. Eng. 2003, 16, 193–198. [Google Scholar] [CrossRef]
- Ayele, A.; Haile, S.; Alemu, D.; Kamaraj, M. Comparative utilization of dead and live fungal biomass for the removal of heavy metal: A concise review. Sci. World J. 2021, 2021, 5588111. [Google Scholar] [CrossRef]
- Sintorini, M.; Widyatmoko, H.; Sinaga, E.; Aliyah, N. Effect of pH on metal mobility in the soil. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Surakarta, Indonesia, 24–25 August 2021; Volume 737, p. 012071. [Google Scholar] [CrossRef]
- White, C.; Sayer, J.; Gadd, G. Microbial solubilization and immobilization of toxic metals: Key biogeochemical processes for treatment of contamination. FEMS Microbiol. Rev. 1997, 20, 503–516. [Google Scholar] [CrossRef]
- Fomina, M.; Hillier, S.; Charnock, J.; Melville, K.; Alexander, I.J.; Gadd, G. Role of oxalic acid overexcretion in transformations of toxic metal minerals by Beauveria caledonica. Appl. Environ. Microbiol. 2005, 71, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Michalak, I.; Chojnacka, K.; Witek-Krowiak, A. State of the art for the biosorption process—A review. Appl. Biochem. Biotechnol. 2013, 170, 1389–1416. [Google Scholar] [CrossRef] [PubMed]
- Filote, C.; Rosca, M.; Hlihor, R.M.; Cozma, P.; Simion, I.M.; Apostol, M.; Gavrilescu, M. Sustainable application of biosorption and bioaccumulation of persistent pollutants in wastewater treatment: Current practice. Processes 2021, 9, 1696. [Google Scholar] [CrossRef]
- Vojtková, H. New strains of copper-resistant pseudomonas bacteria isolated from anthropogenically polluted soils. In Proceedings of the International Multidisciplinary Scientific GeoConference Surveying Geology and Mining Ecology Management, SGEM, Albena, Bulgaria, 17–26 June 2014; Volume 1, pp. 451–457. [Google Scholar] [CrossRef]
- Vojtková, H. Biodiversity of Pseudomonas bacterial strains isolated from Ostrava Lagoons, Czech Republic. In Proceedings of the 15th International Multidisciplinary Scientific Geoconference SGEM 2015, Albena, Bulgaria, 18–24 June 2015; pp. 291–296. [Google Scholar] [CrossRef]
- Xu, X.; Hao, R.; Xu, H.; Lu, A. Removal mechanism of Pb (II) by Penicillium polonicum: Immobilization, adsorption, and bioaccumulation. Sci. Rep. 2020, 10, 9079. [Google Scholar] [CrossRef] [PubMed]
- Paria, K.; Pyne, S.; Chakraborty, S.K. Optimization of heavy metal (lead) remedial activities of fungi Aspergillus penicillioides (F12) through extra cellular polymeric substances. Chemosphere 2022, 286, 131874. [Google Scholar] [CrossRef]
- Antsotegi-Uskola, M.; Markina-Iñarrairaegui, A.; Ugalde, U. New insights into copper homeostasis in filamentous fungi. Int. Microbiol. 2020, 23, 65–73. [Google Scholar] [CrossRef]
- Toledo, H.; Sánchez, C.I.; Marín, L.; Amich, J.; Calera, J.A. Regulation of zinc homeostatic genes by environmental pH in the filamentous fungus Aspergillus fumigatus. Environ. Microbiol. 2022, 24, 643–666. [Google Scholar] [CrossRef]
- Ge, W.; Zamri, D.; Mineyama, H.; Valix, M. Bioaccumulation of heavy metals on adapted Aspergillus foetidus. Adsorption 2011, 17, 901–910. [Google Scholar] [CrossRef]
- Ramya, D.; Kiruba, N.J.M.; Thatheyus, A.J. Biosorption of heavy metals using fungal biosorbents—A review. Fungi Bio-Prospect. Sustain. Agric. Environ. Nano-Technol. 2021, 2, 331–352. [Google Scholar] [CrossRef]
- Gadd, G.M. Interactions of fungi with toxic metals. In The genus Aspergillus: From Taxonomy and Genetics to Industrial Application; Springer: Boston, MA, USA, 1994; pp. 361–374. [Google Scholar]
- Robinson, J.R.; Isikhuemhen, O.S.; Anike, F.N. Fungal–metal interactions: A review of toxicity and homeostasis. J. Fungi 2021, 7, 225. [Google Scholar] [CrossRef]
- Karimi, H. Effect of pH and initial pb (II) concentration on the lead removal efficiency from industrial wastewater using Ca(OH)2. Int. J. Water Wastewater Treat 2017, 3, 1–4. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).