The Detection of E. coli and S. aureus on Sensors without Immobilization by Using Impedance Spectroscopy †
Abstract
:1. Introduction
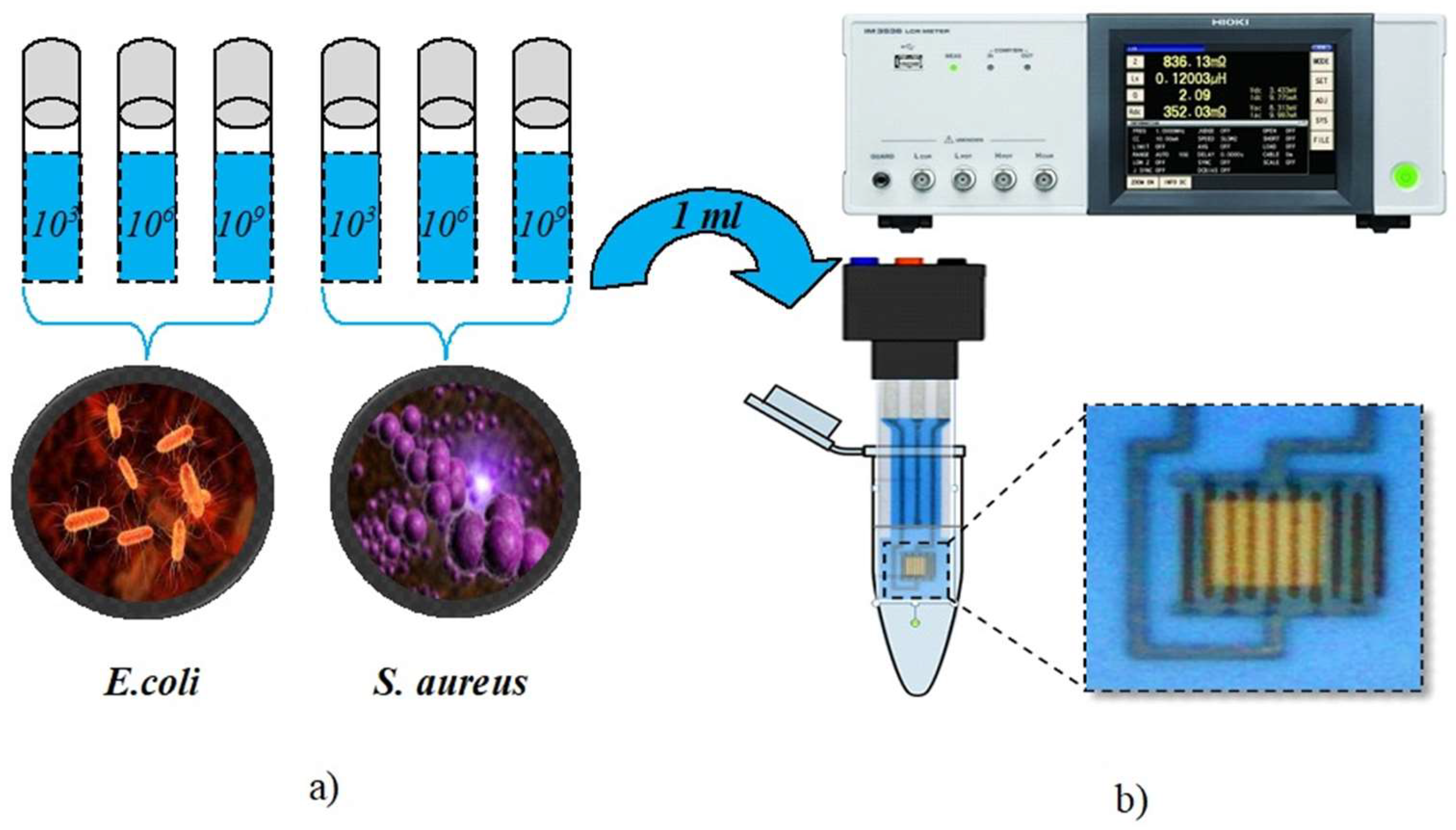
2. Materials and Methods
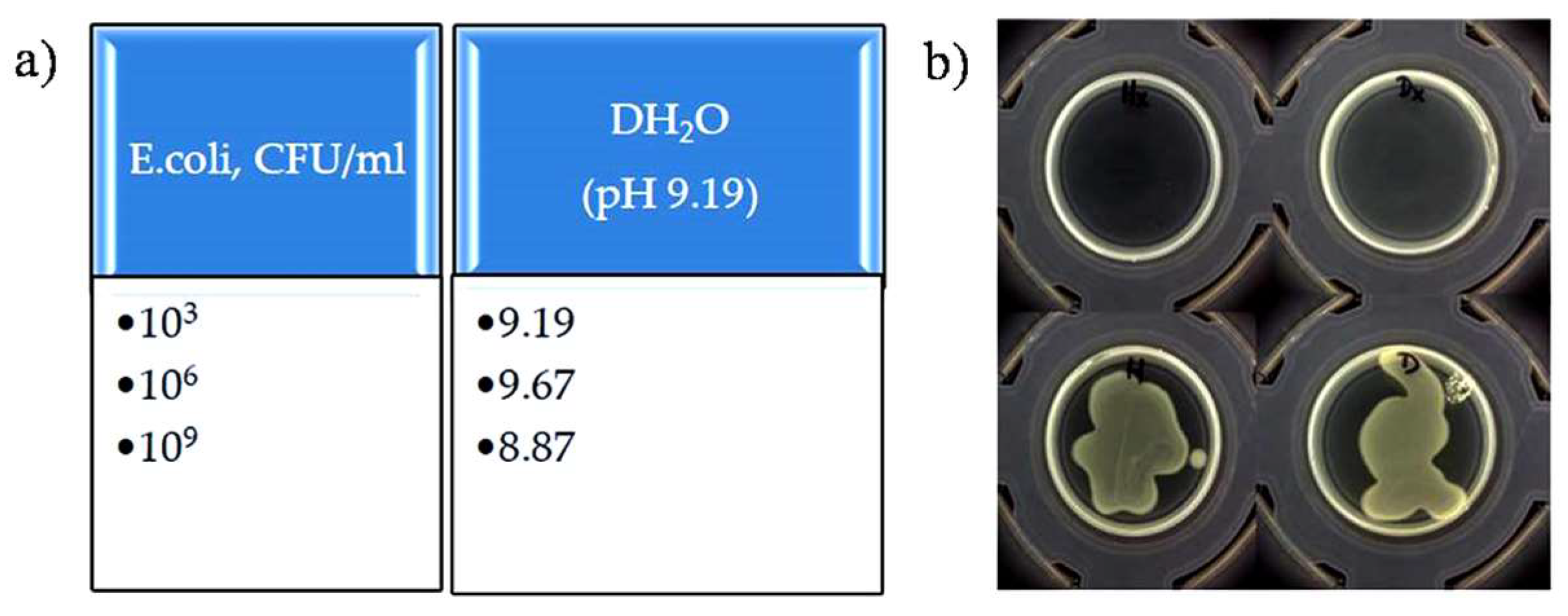
2.1. Preparation of the Biological Samples (Gram-Positive S. aureus and Gram-Negative E. coli)
2.2. Preparation of the IDE Sensor Surface
2.3. Electrical Impedance Measurements
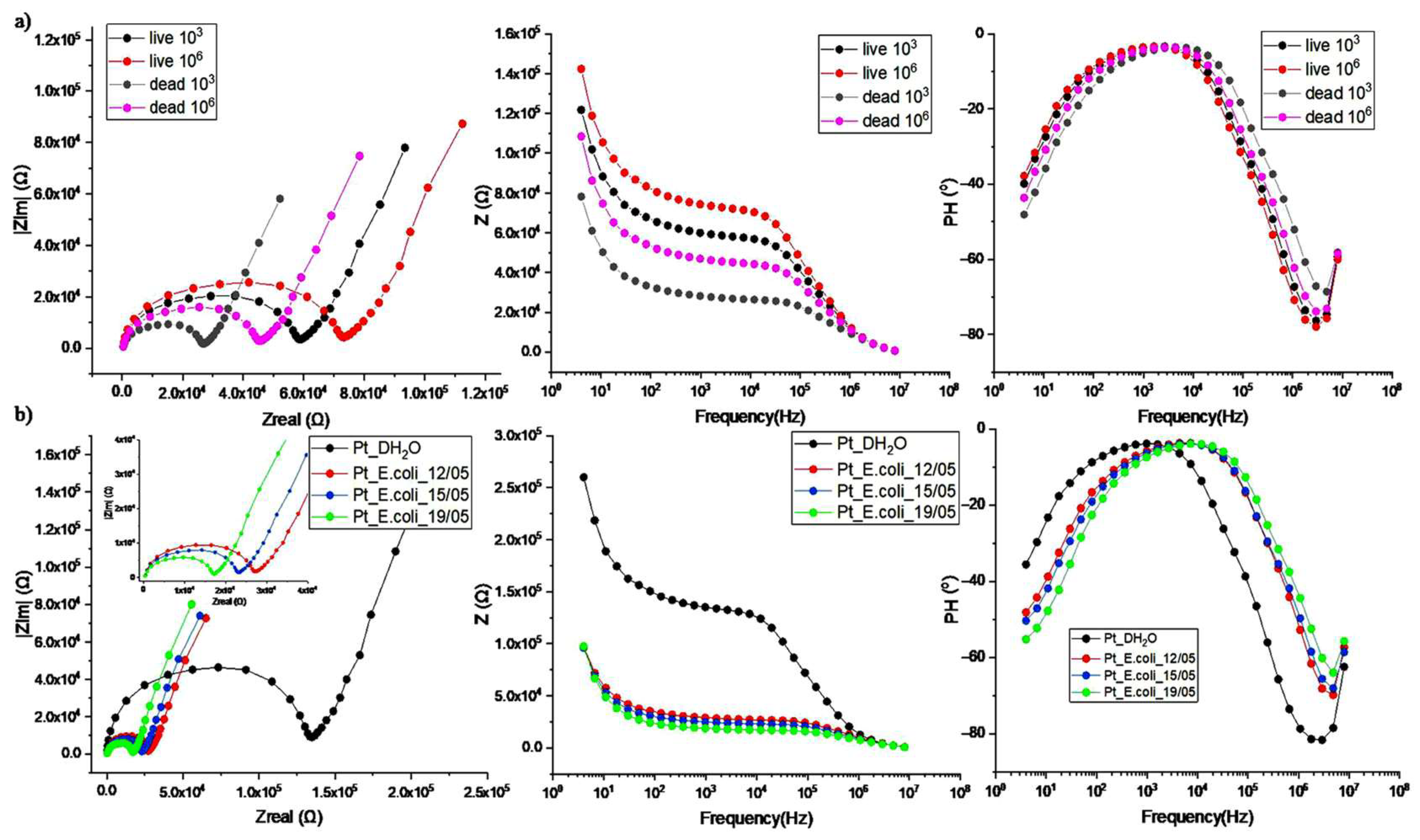
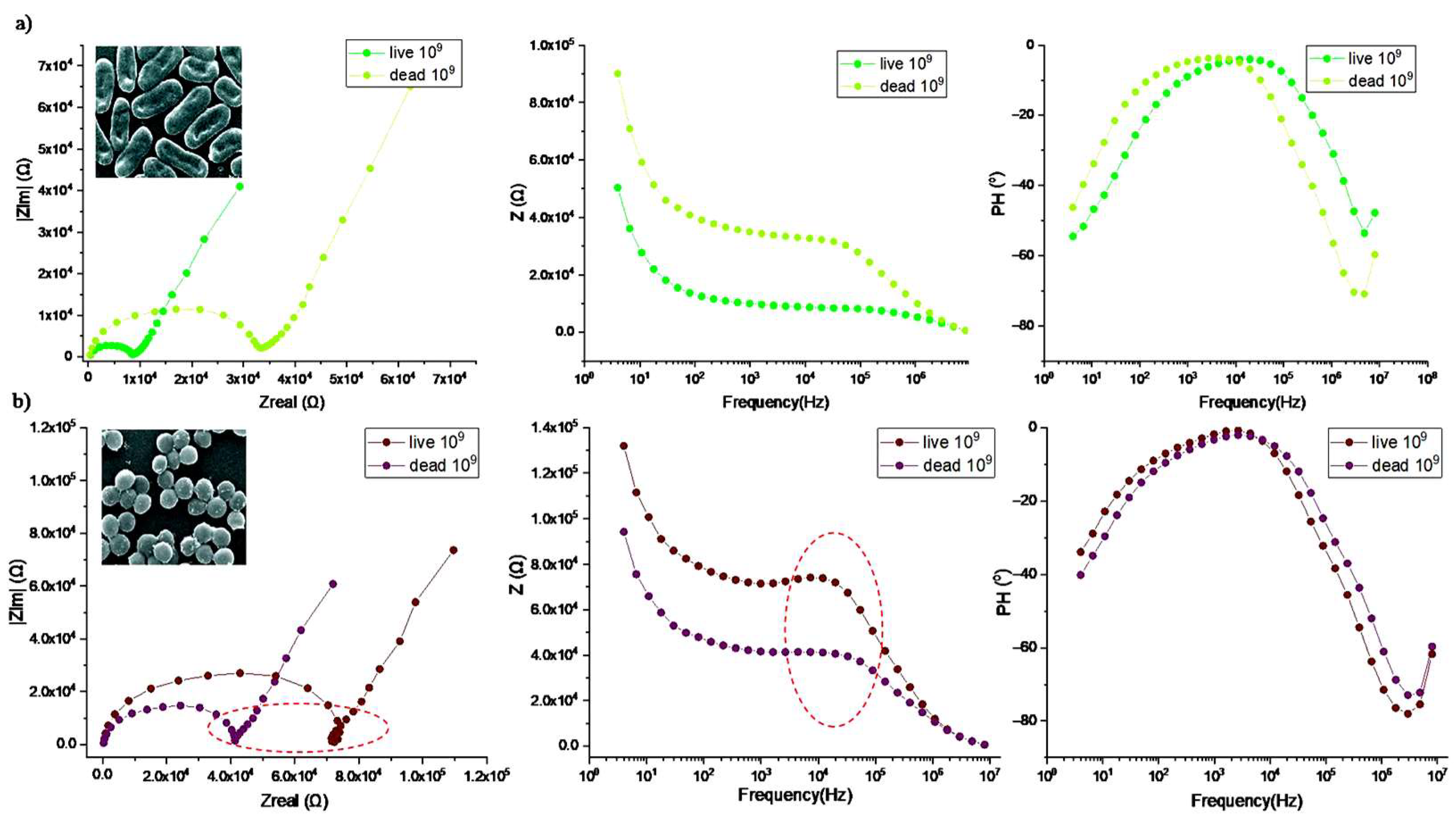
3. Results and Discussion
Detection of Bacteria Cells—Characterization of Impedance Spectrum Data
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mallén-Alberdi, M.; Mas, J.; Fernández-Sánchez, C.; Baldi, A. Impedance spectral fingerprint of E. coli cells on interdigitated electrodes: A new approach for label free and selective detection. Sens. Bio-Sens. Res. 2016, 7, 100–106. [Google Scholar] [CrossRef]
- Wang, R.; Lum, J.; Callaway, Z.; Lin, J.; Bottje, W.; Li, Y. A Label-Free Impedance Immunosensor Using Screen-Printed Interdigitated Electrodes and Magnetic Nanobeads for the Detection of E. coli O157:H7. Biosensors 2015, 5, 791–803. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhou, H.; Hao, H.; Gong, Q.; Nie, K. Detection of Escherichia coli with a label-free impedimetric biosensor based on lectin functionalized mixed self-assembled monolayer. Sens. Actuators B Chem. 2016, 229, 297–304. [Google Scholar] [CrossRef]
- Ahmed, A.; Rushworth, J.V.; Hirst, N.A.; Millner, P.A. Biosensors for Whole-Cell Bacterial Detection. Clin. Microbiol. Rev. 2014, 27, 631–646. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.B.D.; Sporer, C.; Sanvicens, N.; Pascual, N.; Errachid, A.; Martinez, E.; Marco, M.-P.; Teixeira, V.; Samiter, J. Detection of pathogenic Bacteria by Electrochemical Impedance Spectroscopy: Influence of the immobilization strategies on the sensor performance. Procedia Chem. 2009, 1, 1291–1294. [Google Scholar] [CrossRef]
- Brosel-Oliu, S.; Abramova, N.; Uria, N.; Bratov, A. Impedimetric transducers based on interdigitated electrode arrays for bacterial detection—A review. Anal. Chim. Acta 2019, 1088, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Swami, P.; Sharma, A.; Anand, S.; Gupta, S. DEPIS: A combined dielectrophoresis and impedance spectroscopy platform for rapid cell viability and antimicrobial susceptibility analysis. Biosens. Bioelectron. 2021, 182, 113190. [Google Scholar] [CrossRef] [PubMed]
- Jira, J.; Rezek, B.; Kriha, V.; Artemenko, A.; Matolínová, I.; Skakalova, V.; Stenclova, P.; Kromka, A. Inhibition of E. coli Growth by Nanodiamond and Graphene Oxide Enhanced by Luria-Bertani Medium. Nanomaterials 2018, 8, 140. [Google Scholar] [CrossRef] [PubMed]
- Kivirand, K.; Min, M.; Rinken, T. Challenges and Applications of Impedance-Based Biosensors in Water Analysis. In Biosensors for Environmental Monitoring; Rinken, T., Kivirand, K., Eds.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Lanzoni, M.G.M. Data Transformation Algorithm for Reliable Bacterial Concentration Detection Using the Impedance Method. J. Electr. Eng. Electron. Technol. 2014, 3, 1–5. [Google Scholar] [CrossRef]
- Cimafonte, M.; Fulgione, A.; Gaglione, R.; Papaianni, M.; Capparelli, R.; Arciello, A.; Censi, S.B.; Borriello, G.; Velotta, R.; Della Ventura, B. Screen Printed Based Impedimetric Immunosensor for Rapid Detection of Escherichia coli in Drinking Water. Sensors 2020, 20, 274. [Google Scholar] [CrossRef] [PubMed]
- Kundu, A.; Ausaf, T.; Rajasekaran, P.; Rajaraman, S. Multimodal Microfluidic Biosensor with Interdigitated Electrodes (IDE) and Microelectrode Array (MEA) for Bacterial Detection and Identification. In Proceedings of the 2019 20th International Conference on Solid-State Sensors, Actuators and Microsystems & Eurosensors XXXIII (TRANSDUCERS & EUROSENSORS XXXIII), Berlin, Germany, 23–27 June 2019; pp. 1199–1202. [Google Scholar] [CrossRef]
- Singh, V.; Rawal, V.; Lakhanpal, S.; Jain, P.; Dahiya, S.; Tripathi, C.C. Immobilized bacteriophage used for specific detection of E. coli using electrochemical impedance sensing. Int. J. Pharm. Sci. Res. 2015, 6, 3913–3919. [Google Scholar]
- Gehring, A.G.; Albin, D.M.; Bhunia, A.K.; Reed, S.A.; Tu, S.-I.; Uknalis, J. Antibody Microarray Detection of Escherichiacoli O157:H7: Quantification, Assay Limitations, and Capture Efficiency. Anal. Chem. 2006, 78, 6601–6607. [Google Scholar] [CrossRef] [PubMed]
- Al-Jumaili, A.; Zafar, M.A.; Bazaka, K.; Weerasinghe, J.; Jacob, M.V. Bactericidal vertically aligned graphene networks derived from renewable precursor. Carbon Trends 2022, 7, 100157. [Google Scholar] [CrossRef]

| CFU/mL | Live | |||||
|---|---|---|---|---|---|---|
| Rs, Ω (×10−14) | Rct, kΩ | CPE1 (×10−10) | n1 | CPE2 (×10−7) | n2 | |
| 103 | 9.31 | 166 | 5.55 | 0.74 | 5.86 | 0.69 |
| 106 | 9.01 | 80.53 | 7.04 | 0.74 | 7.59 | 0.71 |
| 109 | 8.79 | 41.08 | 8.86 | 0.73 | 9.56 | 0.72 |
| CFU/mL | Dead | |||||
| Rs, Ω (×10−14) | Rct, kΩ | CPE1 (×10−11) | n1 | CPE2 (×10−6) | n2 | |
| 103 | 8.69 | 70.24 | 1.37 | 0.99 | 1.88 | 0,60 |
| 106 | 8.64 | 50.52 | 4.05 | 0.93 | 1.85 | 0.61 |
| 109 | 7.26 | 22.67 | 1.33 | 1.00 | 4.08 | 0.50 |
| 109 CFU/mL | Rs, Ω (×10−14) | Rct, kΩ | CPE1 | n1 | CPE2 (×10−6) | n2 |
|---|---|---|---|---|---|---|
| E. coli | ||||||
| Live | 1.60 | 9.21 | 8.86 × 10−09 | 0.70 | 2.26 | 0.70 |
| Dead | 1.62 | 32.70 | 5.39 × 10−10 | 0.77 | 1.35 | 0.72 |
| S. aureus | ||||||
| Live | 9.59 | 73.46 | 1.36 × 10−10 | 0.85 | 1.23 | 0.70 |
| Dead | 9.58 | 58.37 | 1.73 × 10−10 | 0.83 | 1.40 | 0.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutsul, O.; Rutherford, D.; Barinkova, M.; Slobodyan, V.; Rezek, B. The Detection of E. coli and S. aureus on Sensors without Immobilization by Using Impedance Spectroscopy. Eng. Proc. 2023, 58, 79. https://doi.org/10.3390/ecsa-10-16073
Gutsul O, Rutherford D, Barinkova M, Slobodyan V, Rezek B. The Detection of E. coli and S. aureus on Sensors without Immobilization by Using Impedance Spectroscopy. Engineering Proceedings. 2023; 58(1):79. https://doi.org/10.3390/ecsa-10-16073
Chicago/Turabian StyleGutsul, Oksana, David Rutherford, Marketa Barinkova, Vsevolod Slobodyan, and Bohuslav Rezek. 2023. "The Detection of E. coli and S. aureus on Sensors without Immobilization by Using Impedance Spectroscopy" Engineering Proceedings 58, no. 1: 79. https://doi.org/10.3390/ecsa-10-16073





