Performance Evaluation of Various Ni-Based Catalysts for the Production of Hydrogen via Steam Methane Reforming Process †
Abstract
:1. Introduction
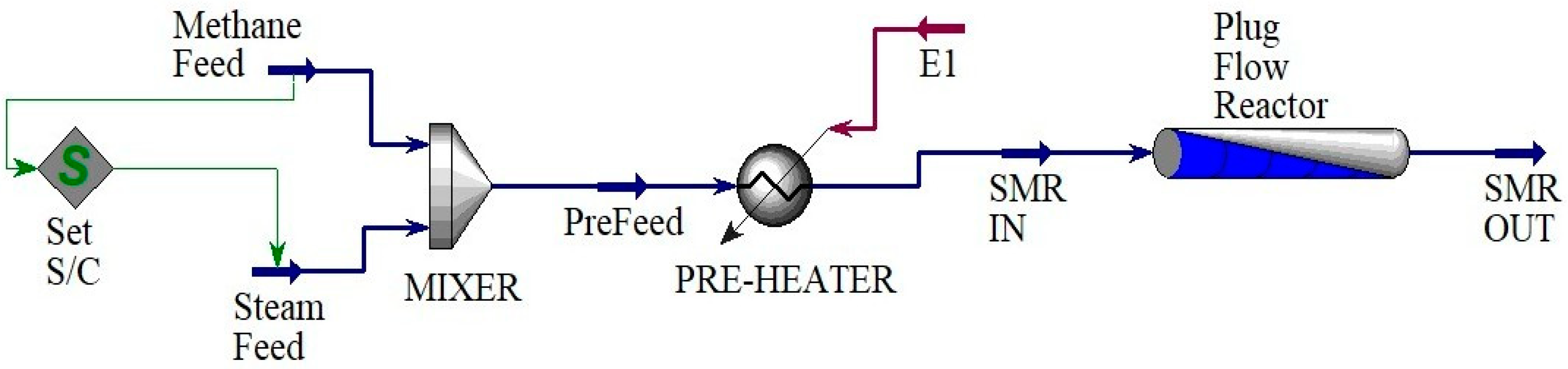
2. Materials and Methods
- 1D heterogeneous plug flow model with adiabatic operation.
- The reactant flow is realistic to the ideal gas law.
- The temperature of the reaction and reactant concentration gradients are negligible in the direction of the radius. As a result, accountable changes in temperature and concentration are only in one direction, specifically in the axial direction.
- The bed porosity and size of catalyst particles are uniform.
- The catalyst particle temperature gradient is neglected.
Modelling and Simulating Steps for Aspen HYSYS
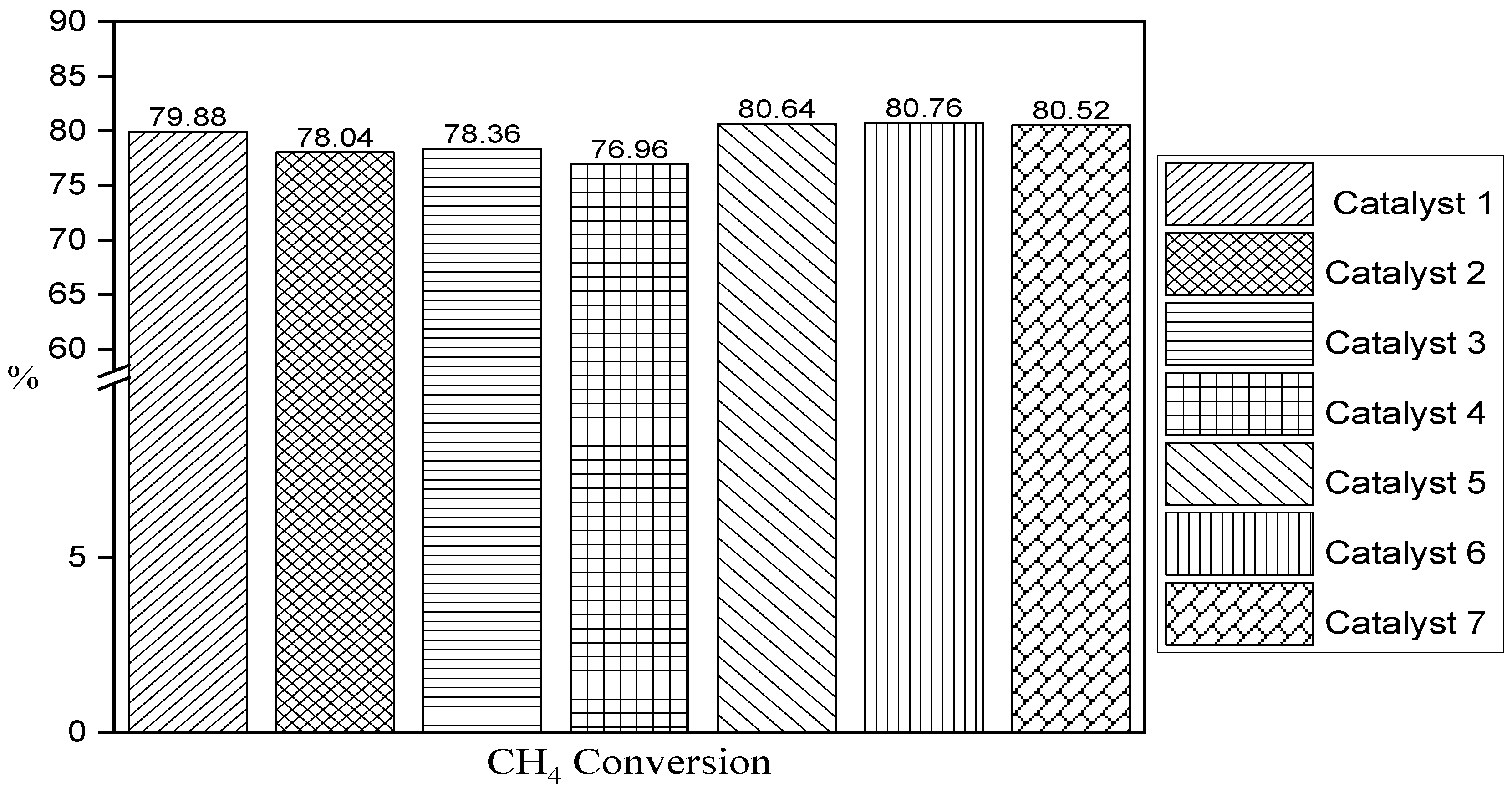
3. Results
4. Discussion
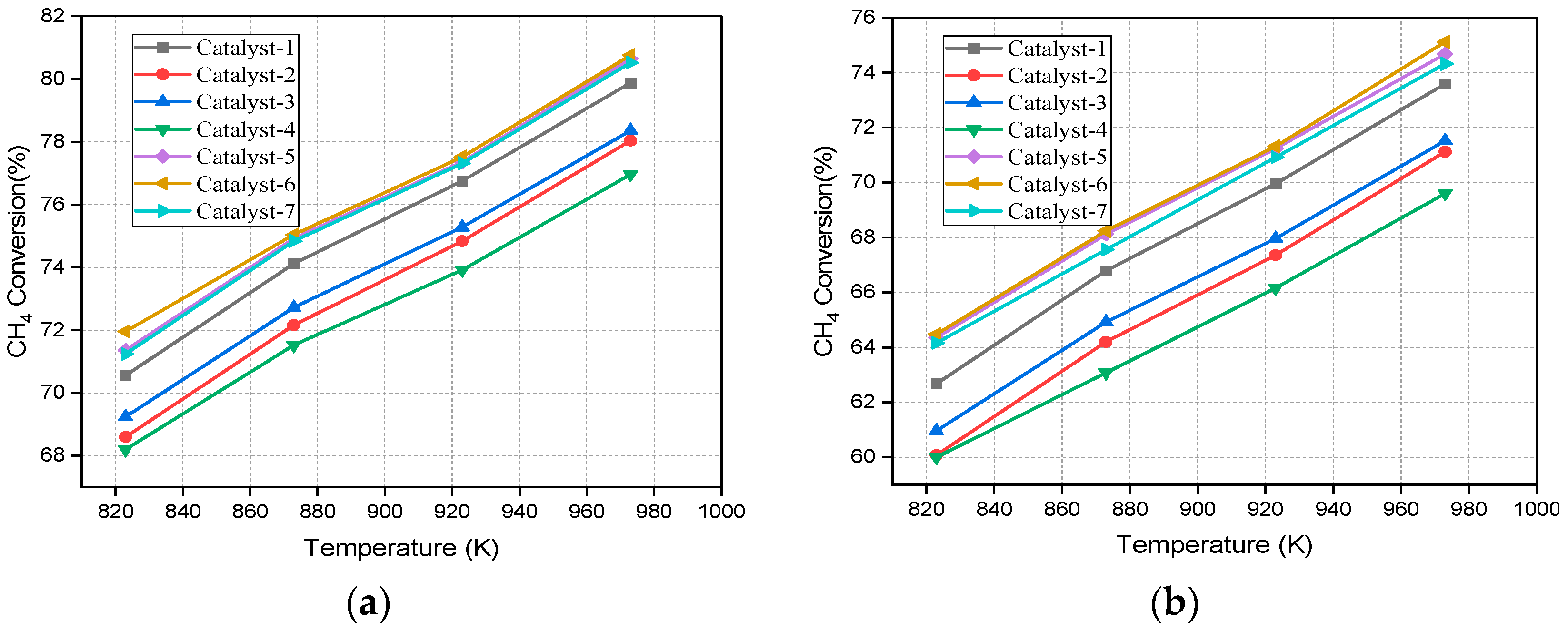
4.1. Effect of Temperature
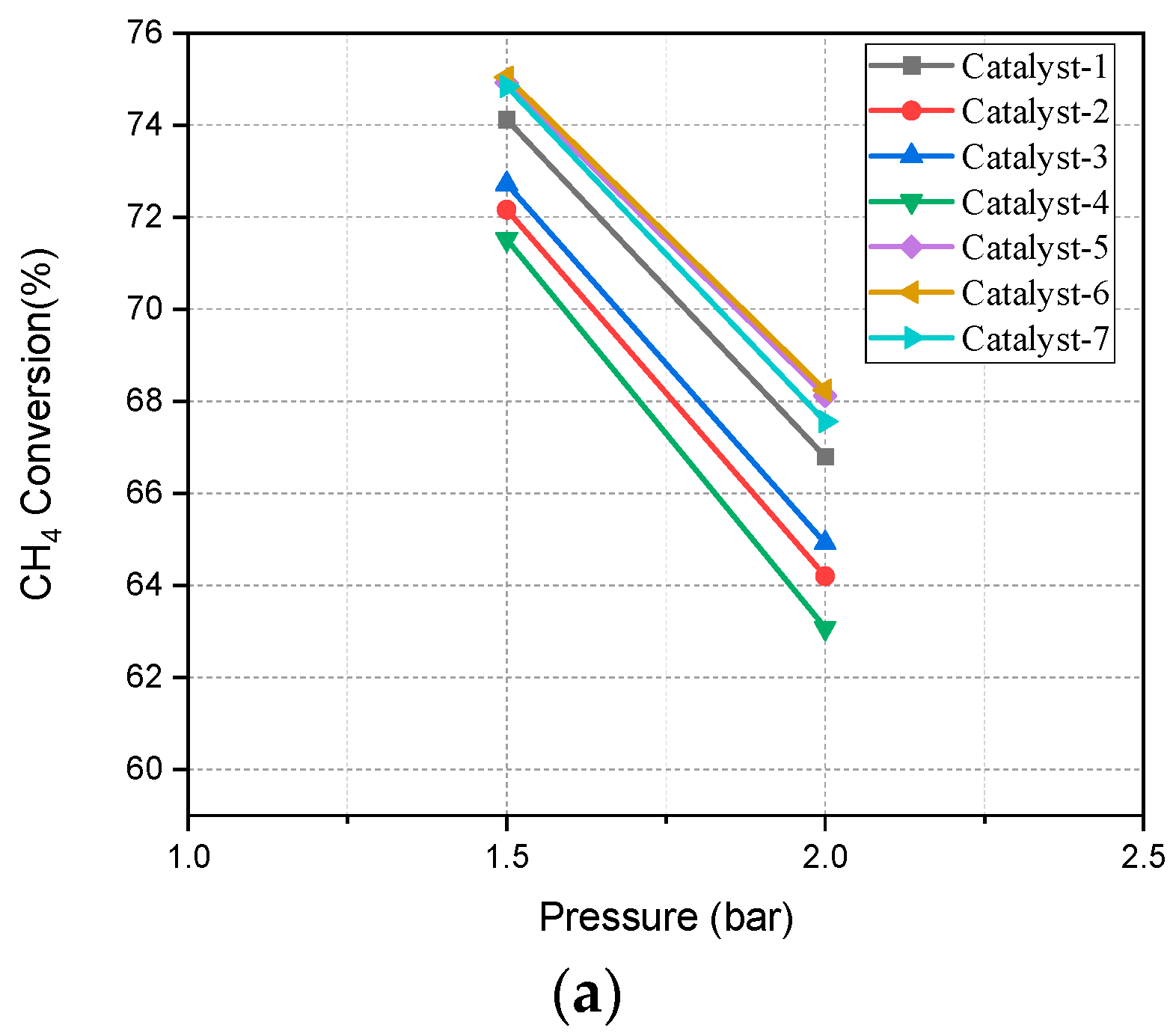
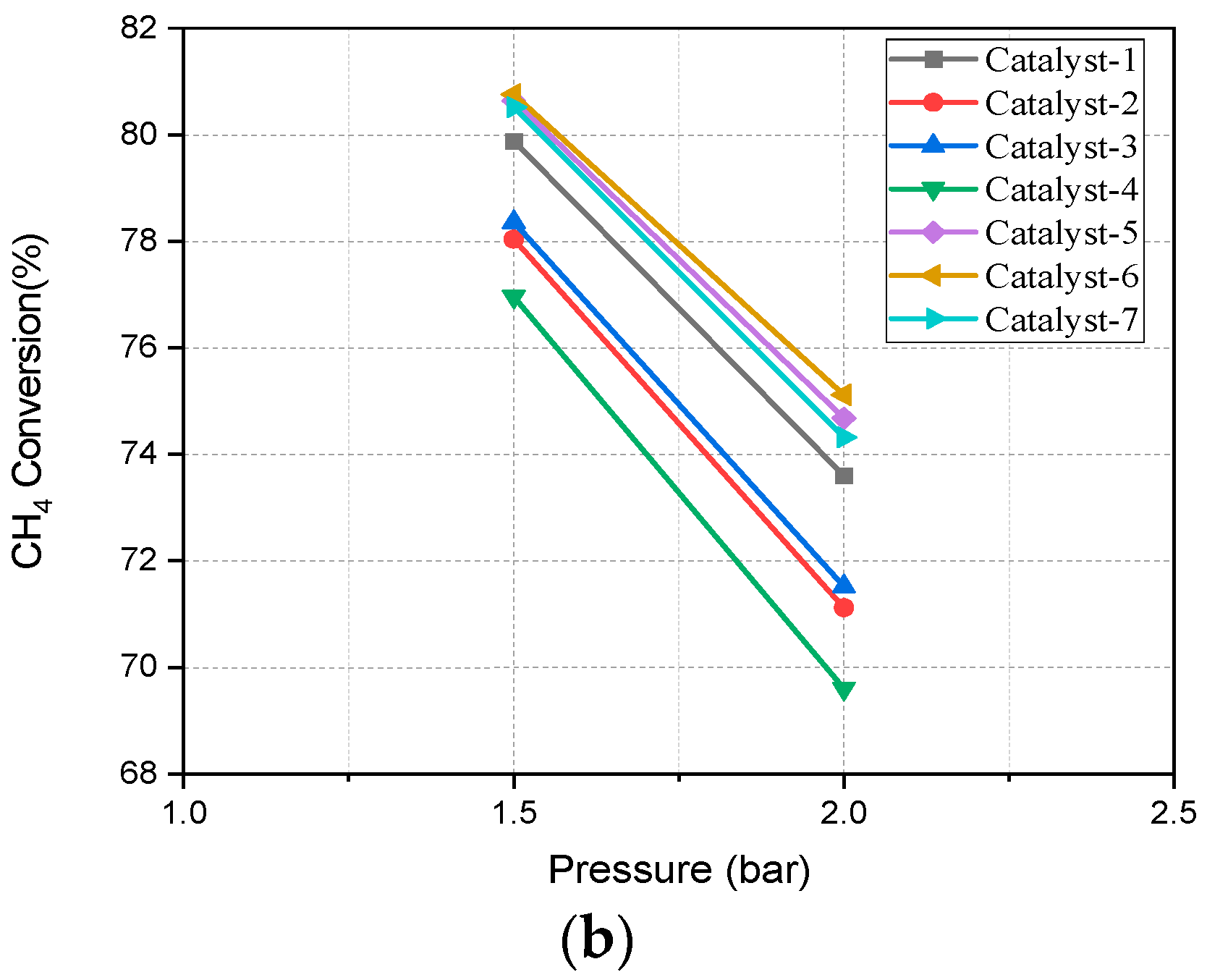
4.2. Pressure Effect on SMR Reaction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borowski, P.F. Mitigating Climate Change and the Development of Green Energy versus a Return to Fossil Fuels Due to the Energy Crisis in 2022. Energies 2022, 15, 9289. [Google Scholar] [CrossRef]
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The role of renewable energy in the global energy transformation. Energy Strategy Rev. 2019, 24, 38–50. [Google Scholar] [CrossRef]
- Kabeyi, M.J.B.; Olanrewaju, O.A. Biogas Production and Applications in the Sustainable Energy Transition. J. Energy 2022, 2022, 8750221. [Google Scholar] [CrossRef]
- Faheem, H.H.; Tanveer, H.U.; Abbas, S.Z.; Maqbool, F. Comparative study of conventional steam-methane-reforming (SMR) and auto-thermal-reforming (ATR) with their hybrid sorption enhanced (SE-SMR & SE-ATR) and environmentally benign process models for the hydrogen production. Fuel 2021, 297, 120769. [Google Scholar] [CrossRef]
- Kalamaras, C.M.; Efstathiou, A.M. Hydrogen Production Technologies: Current State and Future Developments. Conf. Pap. Energy 2013, 2013, 690627. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Z.; Hu, Y.H. Steam reforming of methane: Current states of catalyst design and process upgrading. Renew. Sustain. Energy Rev. 2021, 149, 111330. [Google Scholar] [CrossRef]
- Pareek, A.; Dom, R.; Gupta, J.; Chandran, J.; Adepu, V.; Borse, P.H. Insights into renewable hydrogen energy: Recent advances and prospects. Mater. Sci. Energy Technol. 2020, 3, 319–327. [Google Scholar] [CrossRef]
- Kim, T.-Y.; Lee, J.-H.; Jo, S.; Kim, J.; Woo, J.-H.; Dhanusuraman, R.; Kim, J.-C.; Lee, S.-C. Improving the Stability of Ru-Doped Ni-Based Catalysts for Steam Methane Reforming during Daily Startup and Shutdown Operation. Catalysts 2023, 13, 949. [Google Scholar] [CrossRef]
- Iglesias, I.; Forti, M.; Baronetti, G.; Mariño, F. Zr-enhanced stability of ceria based supports for methane steam reforming at severe reaction conditions. Int. J. Hydrogen Energy 2019, 44, 8121–8132. [Google Scholar] [CrossRef]
- Lee, H.; Lee, D. Synthesis Chemistry and Properties of Ni Catalysts Fabricated on SiC@Al2O3 Core-Shell Microstructure for Methane Steam Reforming. Catalysts 2020, 10, 391. [Google Scholar] [CrossRef]
- Meloni, E.; Martino, M.; Palma, V. A Short Review on Ni Based Catalysts and Related Engineering Issues for Methane Steam Reforming. Catalysts 2020, 10, 352. [Google Scholar] [CrossRef]
- Giwa, A.; Giwa, S.O. Simulation, Sensitivity Analysis and Optimization of Hydrogen Production by Steam Reforming of Methane Using Aspen Plus. Available online: www.ijert.org (accessed on 29 December 2023).
- Moreno, A.; Ramirez-Reina, T.; Ivanova, S.; Roger, A.-C.; Centeno, M.; Odriozola, J.A. Bimetallic Ni–Ru and Ni–Re Catalysts for Dry Reforming of Methane: Understanding the Synergies of the Selected Promoters. Front. Chem. 2021, 9, 694976. [Google Scholar] [CrossRef] [PubMed]
- Ngo, S.I.; Lim, Y.-I.; Kim, W.; Seo, D.J.; Yoon, W.L. Computational fluid dynamics and experimental validation of a compact steam methane reformer for hydrogen production from natural gas. Appl. Energy 2019, 236, 340–353. [Google Scholar] [CrossRef]
- Upadhyay, M.; Lee, H.; Kim, A.; Lee, S.-H.; Lim, H. CFD simulation of methane steam reforming in a membrane reactor: Performance characteristics over range of operating window. Int. J. Hydrogen Energy 2021, 46, 30402–30411. [Google Scholar] [CrossRef]
- Rahman, Z.U.; Ahmad, I.; Kano, M.; Mustafa, J. Model Development and Exergy Analysis of a Microreactor for the Steam Methane Reforming Process in a CFD Environment. Entropy 2019, 21, 399. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.Z.; Dupont, V.; Mahmud, T. Kinetics study and modelling of steam methane reforming process over a NiO/Al2O3 catalyst in an adiabatic packed bed reactor. Int. J. Hydrogen Energy 2017, 42, 2889–2903. [Google Scholar] [CrossRef]
- Xu, J.; Froment, G.F. Methane Steam Reforming, Methanation and Water-Gas Shift: 1. Intrinsic Kinetics. AIChE J. 1989, 35, 88–96. [Google Scholar] [CrossRef]
- Vogl, V. CFD Simulation of a Methane Steam Reformer for the Steam-Iron Process. Master’s Thesis, Graz University of Technology, Styria, Austria, December 2016. [Google Scholar]
- de Medeiros, J.P.F.; Dias, V.d.F.; da Silva, J.M.; da Silva, J.D. Thermochemical Performance Analysis of the Steam Reforming of Methane in a Fixed Bed Membrane Reformer: A Modelling and Simulation Study. Membranes 2021, 11, 6. [Google Scholar] [CrossRef] [PubMed]
| S. No. | Catalyst and Modifications | Key Findings |
|---|---|---|
| 1 | Ni/SiO2Al2O3 |
|
| 2 | Ni0.03Mg0.97O solid solution |
|
| 3 | Zinc addition to Ni/Al2O3 |
|
| 4 | Cu addition to Ni/Al2O3 |
|
| 5 | 15Ni-1Cu-5Zn/γ- Al2O3 |
|
| 6 | K as a promoter to Ni/Al2O3 |
|
| 7 | Ni-0309S/γ- Al2O3 |
|
| Catalyst | Name | dp (m) | ρcat (kg/m3) | ρbed (kg/m3) | εb |
|---|---|---|---|---|---|
| Catalyst—1 | Ni/MgAl2O4 | 2.00 × 10−3 | 1870 | 1122 | 0.4 |
| Catalyst—2 | Ni-0309S/γ-Al2O3 | 1.75 × 10−3 | 3737 | 2429 | 0.35 |
| Catalyst—3 | Ni/K1.4Al10.9O17.2 | 5.60 × 10−3 | 1687 | 877 | 0.48 |
| Catalyst—4 | 18 wt% NiO/α-Al2O3 | 1.20 × 10−3 | 1870 | 1122 | 0.4 |
| Catalyst—5 | 15.4 wt% Ni/α-Al2O3 | 1.60 × 10−3 | 1274 | 726 | 0.43 |
| Catalyst—6 | 10.34 wt% Ni/α-Al2O3 | 2.15 × 10−4 | 2200 | 1320 | 0.4 |
| Catalyst—7 | 8.6 wt% Ni/γ-Al2O3 | 5.40 × 10−3 | 2355 | 1154 | 0.51 |
| Catalyst | E1 (J/mol) | E2 (J/mol) | E3 (J/mol) | A1 (mol bar0.5/kgcat s) | A2 (mol/kgcat bar s) | A3 (mol bar0.5/kgcat s) |
|---|---|---|---|---|---|---|
| Catalyst—1 | 240,100 | 67,130 | 243,900 | 1.17 × 1015 | 5.43 × 105 | 2.83 × 1014 |
| Catalyst—2 | 209,500 | 70,200 | 211,500 | 9.048 × 1011 | 5.43 × 105 | 2.14 × 109 |
| Catalyst—3 | 218,550 | 73,523 | 236,850 | 5.83 × 1011 | 2.51 × 104 | 4.67 × 1013 |
| Catalyst—4 | 257,010 | 89,230 | 236,700 | 5.19 × 1012 | 9.90 × 106 | 1.32 × 1013 |
| Catalyst—5 | 217,010 | 68,200 | 215,840 | 5.79 × 1012 | 9.33 × 106 | 1.29 × 1013 |
| Catalyst—6 | 216,722 | 67,966 | 227,941 | 9.78 × 1014 | 5.29 × 105 | 2.57 × 1014 |
| Catalyst—7 | 240,100 | 67,130 | 243,900 | 9.49 × 1015 | 4.39 × 106 | 2.29 × 1015 |
| Temperature (K) | Author [17] | Present Work | Absolute Error (%) |
|---|---|---|---|
| CH4 Conversion (%) | |||
| 873 | 87.09 | 90.328 | 3.72 |
| 923 | 95.92 | 92.7310404 | 3.32 |
| 973 | 97.88 | 95.0713494 | 2.87 |
| 1023 | 98.88 | 97.144423 | 1.75 |
| 1073 | 99.73 | 98.9914669 | 0.74 |
| 1123 | 99.89 | 99.9374718 | 0.05 |
| 1173 | 99.99 | 99.999617 | 0.01 |
| 1223 | 99.97 | 99.9998265 | 0.03 |
| 1273 | 99.74 | 99.9998411 | 0.26 |
| Mole Fractions of Products at the Outlet of the Reactor | |||||||||
| P = 10 bar S/C = 3 | 773 K | 873 K | 973 K | 1073 K | |||||
| Exit | This work | [4] | This work | [4] | This work | [4] | This work | [4] | |
| CH4 | 0.265 | 0.26 | 0.206 | 0.203 | 0.132 | 0.126 | 0.065 | 0.05 | |
| CO | 0.002 | 0.004 | 0.014 | 0.015 | 0.053 | 0.061 | 0.108 | 0.115 | |
| H2 | 0.166 | 0.174 | 0.291 | 0.3 | 0.414 | 0.434 | 0.528 | 0.563 | |
| H2O | 0.526 | 0.524 | 0.429 | 0.421 | 0.338 | 0.314 | 0.246 | 0.222 | |
| CO2 | 0.041 | 0.038 | 0.06 | 0.061 | 0.063 | 0.065 | 0.053 | 0.05 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subramanya, S.N.; Reddy, V.S.C.; Madav, V. Performance Evaluation of Various Ni-Based Catalysts for the Production of Hydrogen via Steam Methane Reforming Process. Eng. Proc. 2023, 59, 138. https://doi.org/10.3390/engproc2023059138
Subramanya SN, Reddy VSC, Madav V. Performance Evaluation of Various Ni-Based Catalysts for the Production of Hydrogen via Steam Methane Reforming Process. Engineering Proceedings. 2023; 59(1):138. https://doi.org/10.3390/engproc2023059138
Chicago/Turabian StyleSubramanya, Sudeep Noorambala, Vaka Sai Charan Reddy, and Vasudeva Madav. 2023. "Performance Evaluation of Various Ni-Based Catalysts for the Production of Hydrogen via Steam Methane Reforming Process" Engineering Proceedings 59, no. 1: 138. https://doi.org/10.3390/engproc2023059138





