A Study on the Suitability of Constant Boundary Elements for the Simulation of Biological Organs †
Abstract
:1. Introduction
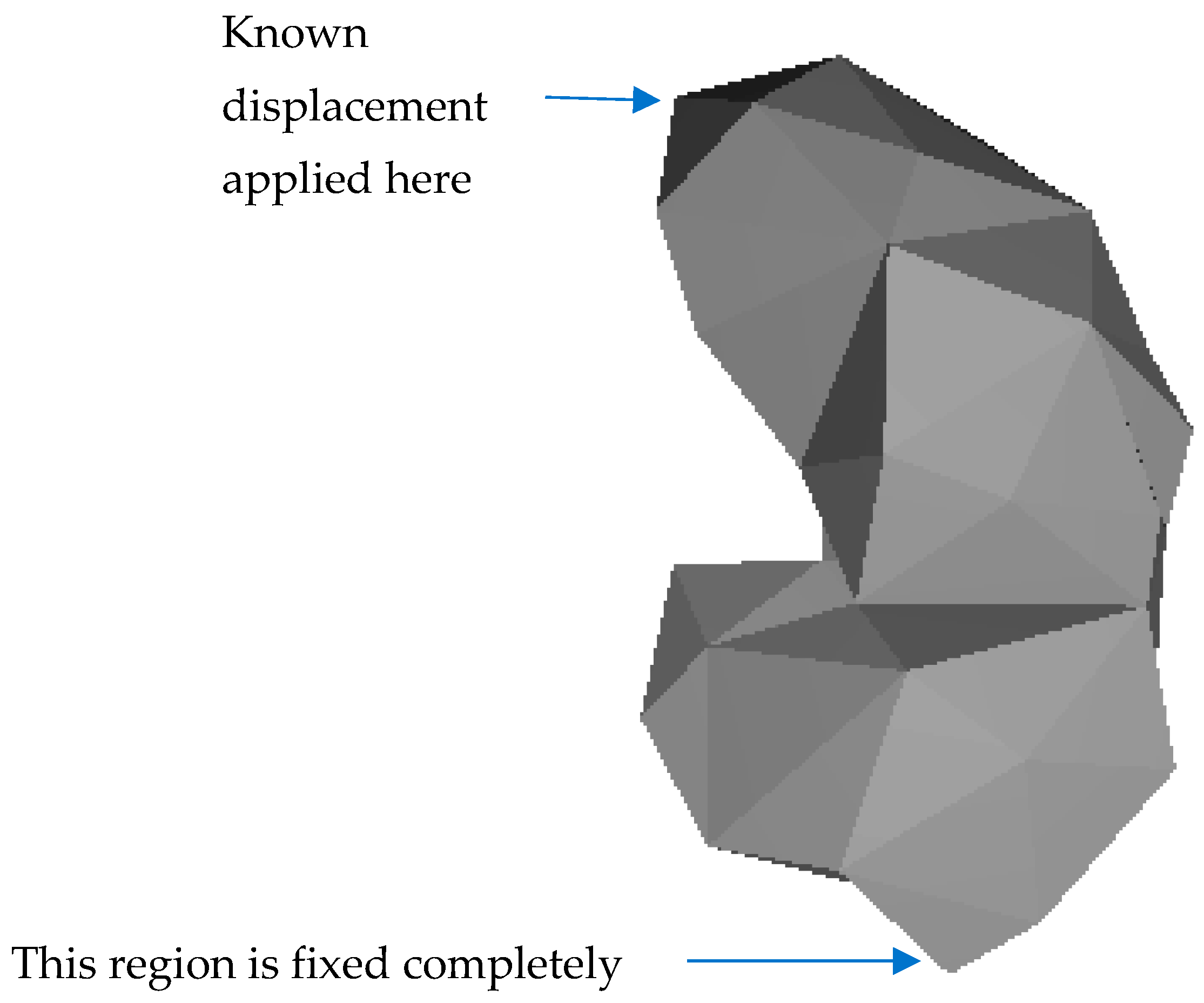
2. Methodology
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, M.; Coleman, M.; McGrath, J. Developing basic hand-eye coordination skills for laparoscopic surgery using gaze training. BJU Int. 2010, 105, 1356–1358. [Google Scholar] [CrossRef] [PubMed]
- Topalli, D.; Cagiltay, N.E. Eye-Hand Coordination Patterns of Intermediate and Novice Surgeons in a Simulation-Based Endoscopic Surgery Training Environment. J. Eye Mov. Res. 2018, 11. [Google Scholar] [CrossRef] [PubMed]
- TraumaMan Surgical Simulator. Available online: https://simulab.com/products/traumaman-surgical-simulator (accessed on 28 July 2023).
- Badash, I.; Burtt, K.; Solorzano, C.A.; Carey, J.N. Innovations in surgery simulation: A review of past, current and future techniques. Ann. Transl. Med. 2016, 4, 453. [Google Scholar] [CrossRef] [PubMed]
- Surgical Simulation. Available online: https://www.caehealthcare.com/surgical-simulation/ (accessed on 28 July 2023).
- Surgical Simulation. Available online: https://www.healthysimulation.com/surgical-simulation/ (accessed on 28 July 2023).
- Simulation Software for Surgical Training and Education. Available online: https://www.insimo.com/ (accessed on 28 July 2023).
- Simbionix Simulators. Available online: https://simbionix.com/simulators/ (accessed on 28 July 2023).
- Surgery Simulators. Available online: https://www.medicalexpo.com/medical-manufacturer/surgery-simulator-44251.html (accessed on 28 July 2023).
- Chen, X.; Hu, J. A review of haptic simulator for oral and maxillofacial surgery based on virtual reality. Expert Rev. Med. Devices 2018, 15, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Coveney, P.V.; Hoekstra, A.; Rodriguez, B.; Viceconti, M. Computational biomedicine. Part II: Organs and systems. Interface Focus 2021, 11, 20200082. [Google Scholar] [CrossRef]
- Sui, Y.; Pan, J.J.; Qin, H.; Liu, H.; Lu, Y. Real-time simulation of soft tissue deformation and electrocautery procedures in laparoscopic rectal cancer radical surgery. Int. J. Med. Robot. 2017, 13, e1827. [Google Scholar] [CrossRef] [PubMed]
- Sacks, M.S.; Motiwale, S.; Goodbrake, C.; Zhang, W. Neural Network Approaches for Soft Biological Tissue and Organ Simulations. J. Biomech. Eng. 2022, 144, 121010. [Google Scholar] [CrossRef] [PubMed]
- Villard, P.i.; Koenig, N.; Perrenot, C.; Perez, M.; Boshier, P. Toward a realistic simulation of organ dissection. Stud. Health Technol. Inf. 2014, 196, 452–456. [Google Scholar]
- Mendizabal, A.; Tagliabue, E.; Hoellinger, T.; Brunet, J.N.; Nikolaev, S.; Cotin, S. Data-driven simulation for augmented surgery. Dev. Nov. Approaches Biomech. Metamater. 2020, 132, 71–96. [Google Scholar]
- Delingette, H. Toward realistic soft-tissue modeling in medical simulation. Proc. IEEE 1998, 86, 512–523. [Google Scholar] [CrossRef]
- Moreno-Guerra, M.R.; Martínez-Romero, O.; Palacios-Pineda, L.M.; Olvera-Trejo, D.; Diaz-Elizondo, J.A.; Flores-Villalba, E.; da Silva, J.V.L.; Elías-Zúñiga, A.; Rodriguez, C.A. Soft Tissue Hybrid Model for Real-Time Simulations. Polymers 2022, 14, 1407. [Google Scholar] [CrossRef] [PubMed]
- Meier, U.; Lopez, O.; Monserrat, C.; Juan, M.C.; Alcaniz, M. Real-time deformable models for surgery simulation: A survey. Comput. Methods Programs Biomed. 2005, 77, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, Y.; Zheng, W.; Yang, B. Real-time Simulation of Virtual Palpation System. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; Volume 234. [Google Scholar] [CrossRef]
- Zhang, J.; Zhong, Y.; Gu, C. Deformable models for surgical simulation: A survey. IEEE Rev. Biomed. Eng. 2018, 11, 143–164. [Google Scholar] [CrossRef] [PubMed]
- Kirana Kumara, P. A Comparison between the Results from Linear Analysis and Nonlinear Analysis in the Context of Simulation of Biological Materials. J. Compos. Sci. 2023, 7, 109. [Google Scholar] [CrossRef]
- Kirana Kumara, P. Studies on the Viability of the Boundary Element Method for the Real-Time Simulation of Biological Organs. Ph.D. Thesis, Centre for Product Design & Manufacturing, Indian Institute of Science, Bangalore, India, 2016. [Google Scholar]
- Kirana Kumara, P. Codes for Solving Three Dimensional Linear Elastostatic Problems Using Constant Boundary Elements While Ignoring Body Forces. Available online: https://eprints.iisc.ac.in/48088/ (accessed on 28 July 2023).
- VHP, n.d., Visible Human Project. Available online: http://www.nlm.nih.gov/research/visible/visible_human.html (accessed on 28 July 2023).
- Kirana Kumara, P. Extracting Three Dimensional Surface Model of Human Kidney from the Visible Human Data Set using Free Software. Leonardo Electron. J. Pract. Technol. 2012, 11, 115–126. [Google Scholar]
- Rasband, W.S. ImageJ. U. S. National Institutes of Health, Bethesda, Maryland, USA. 1997–2018. Available online: https://imagej.nih.gov/ij/ (accessed on 28 July 2023).
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Yushkevich, P.A.; Piven, J.; Hazlett, H.C.; Smith, R.G.; Ho, S.; Gee, J.C.; Gerig, G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 2006, 31, 1116–1128. [Google Scholar] [CrossRef] [PubMed]
- Cignoni, P.; Callieri, M.; Corsini, M. MeshLab: An Open-Source Mesh Processing Tool. In Proceedings of the Sixth Eurographics Italian Chapter Conference, Salerno, Italy, 2–4 July 2008; pp. 129–136. [Google Scholar]
- Rhinoceros. Available online: https://www.rhino3d.com/ (accessed on 28 July 2023).
- Monserrat, C.; Meier, U.; Alcaniz, M.; Chinesta, F.; Juan, M.C. A new approach for the real-time simulation of tissue deformations in surgery simulation. Comput. Methods Programs Biomed. 2001, 64, 77–85. [Google Scholar] [CrossRef] [PubMed]
| Point | Displacement Vector Sum from Nonlinear FEM Analysis (mm) | Displacement Vector Sum from Linear BEM Analysis (mm) | Difference (mm) |
|---|---|---|---|
| 1 | 4.176 | 3.911 | −0.265 |
| 2 | 5.485 | 4.513 | −0.972 |
| 3 | 5.161 | 4.932 | −0.229 |
| 4 | 5.177 | 4.133 | −1.044 |
| 5 | 2.200 | 1.904 | −0.296 |
| 6 | 0.000 | 0.000 | 0 |
| 7 | 0.728 | 0.852 | 0.124 |
| 8 | 0.750 | 0.933 | 0.183 |
| 9 | 1.384 | 1.549 | 0.165 |
| 10 | 1.551 | 1.625 | 0.074 |
| 11 | 1.870 | 1.723 | −0.147 |
| Point | Displacement Vector Sum from Nonlinear FEM Analysis (mm) | Displacement Vector Sum from Linear BEM Analysis (mm) | Difference (mm) |
|---|---|---|---|
| 1 | 5.043 | 3.025 | −2.018 |
| 2 | 6.048 | 3.826 | −2.222 |
| 3 | 5.932 | 4.322 | −1.61 |
| 4 | 5.251 | 4.115 | −1.136 |
| 5 | 2.323 | 2.404 | 0.081 |
| 6 | 0.000 | 0.000 | 0 |
| 7 | 0.661 | 0.150 | −0.511 |
| 8 | 0.984 | 0.162 | −0.822 |
| 9 | 1.373 | 0.389 | −0.984 |
| 10 | 1.584 | 0.546 | −1.038 |
| 11 | 1.767 | 0.839 | −0.928 |
| Point | Displacement Vector Sum from Nonlinear FEM Analysis (mm) | Displacement Vector Sum from Linear BEM Analysis (mm) | Difference (mm) |
|---|---|---|---|
| 1 | 7.551 | 3.707 | −3.844 |
| 2 | 8.922 | 3.775 | −5.147 |
| 3 | 9.231 | 3.838 | −5.393 |
| 4 | 8.446 | 3.389 | −5.057 |
| 5 | 3.146 | 1.717 | −1.429 |
| 6 | 0.000 | 0.000 | 0 |
| 7 | 0.948 | 0.747 | −0.201 |
| 8 | 1.488 | 0.792 | −0.696 |
| 9 | 1.610 | 0.999 | −0.611 |
| 10 | 1.776 | 1.096 | −0.68 |
| 11 | 2.051 | 1.183 | −0.868 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
P, K.K. A Study on the Suitability of Constant Boundary Elements for the Simulation of Biological Organs. Eng. Proc. 2023, 59, 169. https://doi.org/10.3390/engproc2023059169
P KK. A Study on the Suitability of Constant Boundary Elements for the Simulation of Biological Organs. Engineering Proceedings. 2023; 59(1):169. https://doi.org/10.3390/engproc2023059169
Chicago/Turabian StyleP, Kirana Kumara. 2023. "A Study on the Suitability of Constant Boundary Elements for the Simulation of Biological Organs" Engineering Proceedings 59, no. 1: 169. https://doi.org/10.3390/engproc2023059169






