Intraocular Pressure Monitoring System for Glaucoma Patients Using IoT and Machine Learning †
Abstract
1. Introduction
2. Literature Survey
2.1. Existing Systems
2.2. Problems in Existing Systems
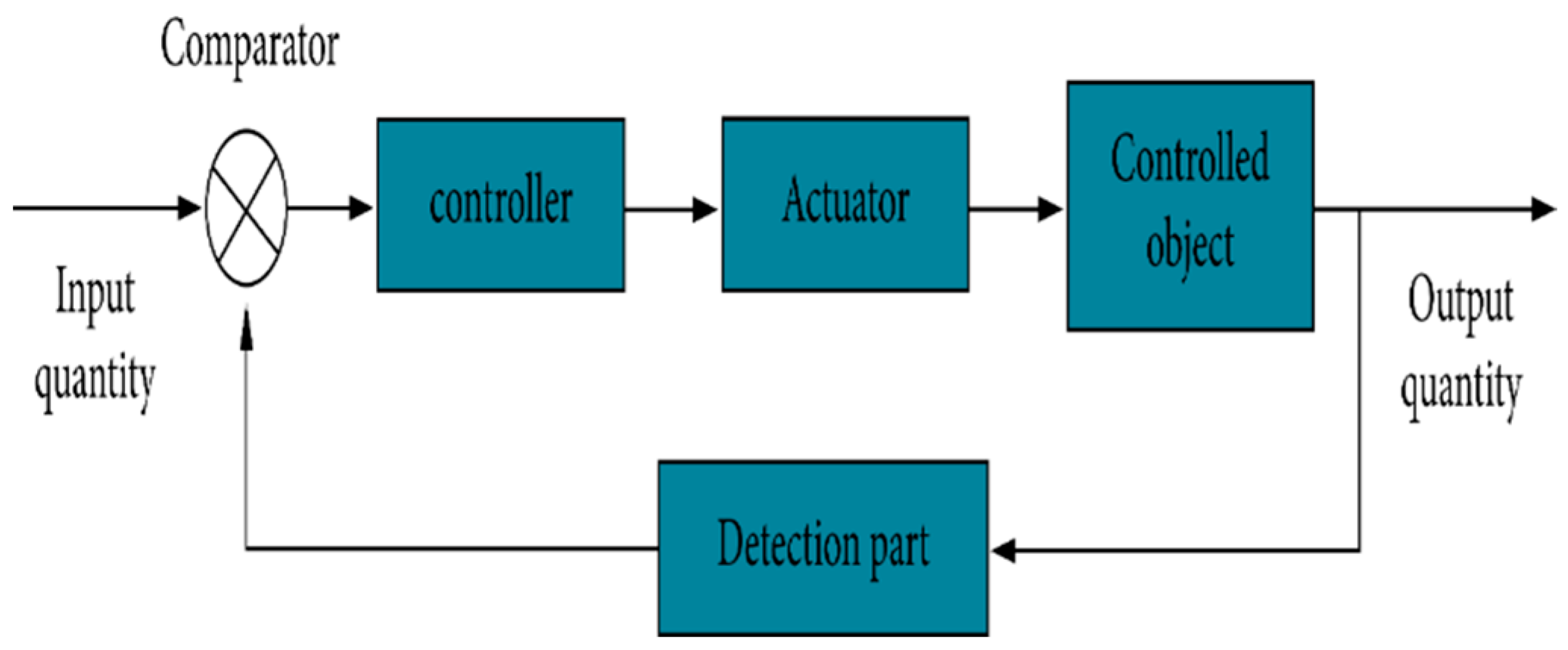
3. Methods/Methodology
3.1. Proposed System
- Input Module: The input module is responsible for receiving input signals related to intraocular pressure. This may involve capturing data from external devices or sensors that measure the pressure within the eye. The input signals are then forwarded to the Microcontroller for further processing.
- Microcontroller: It may include data processing algorithms, calibration routines, and decision-making capabilities. The microcontroller plays a crucial role in managing and controlling the overall functioning of the system.
- Sensor: The sensor utilizes various technologies, such as strain gauges, capacitive sensors, or optical sensors, to detect and quantify the pressure within the eye. The sensor converts the physical pressure into an electrical signal, which is then transmitted to the microcontroller for analysis.
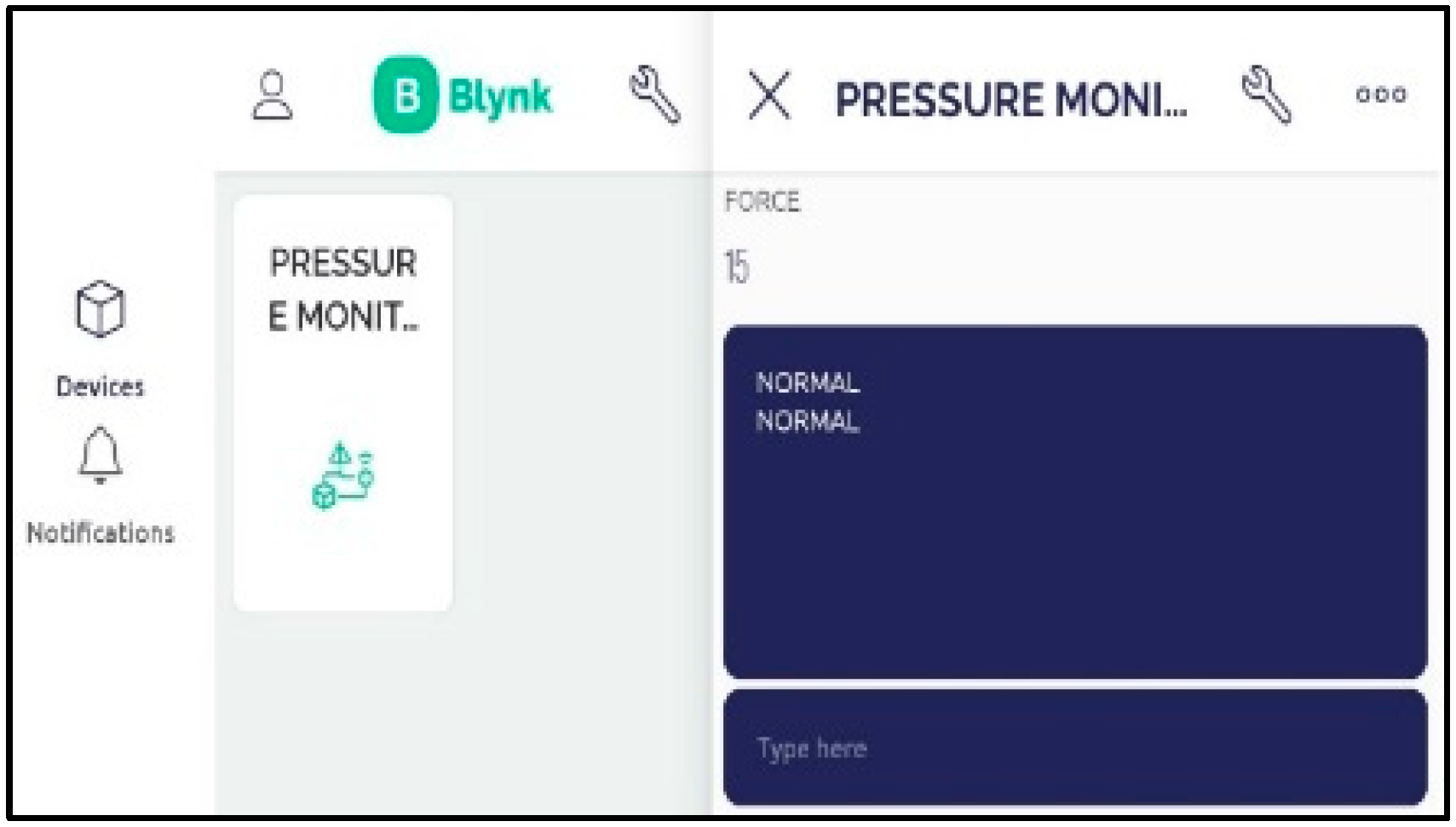
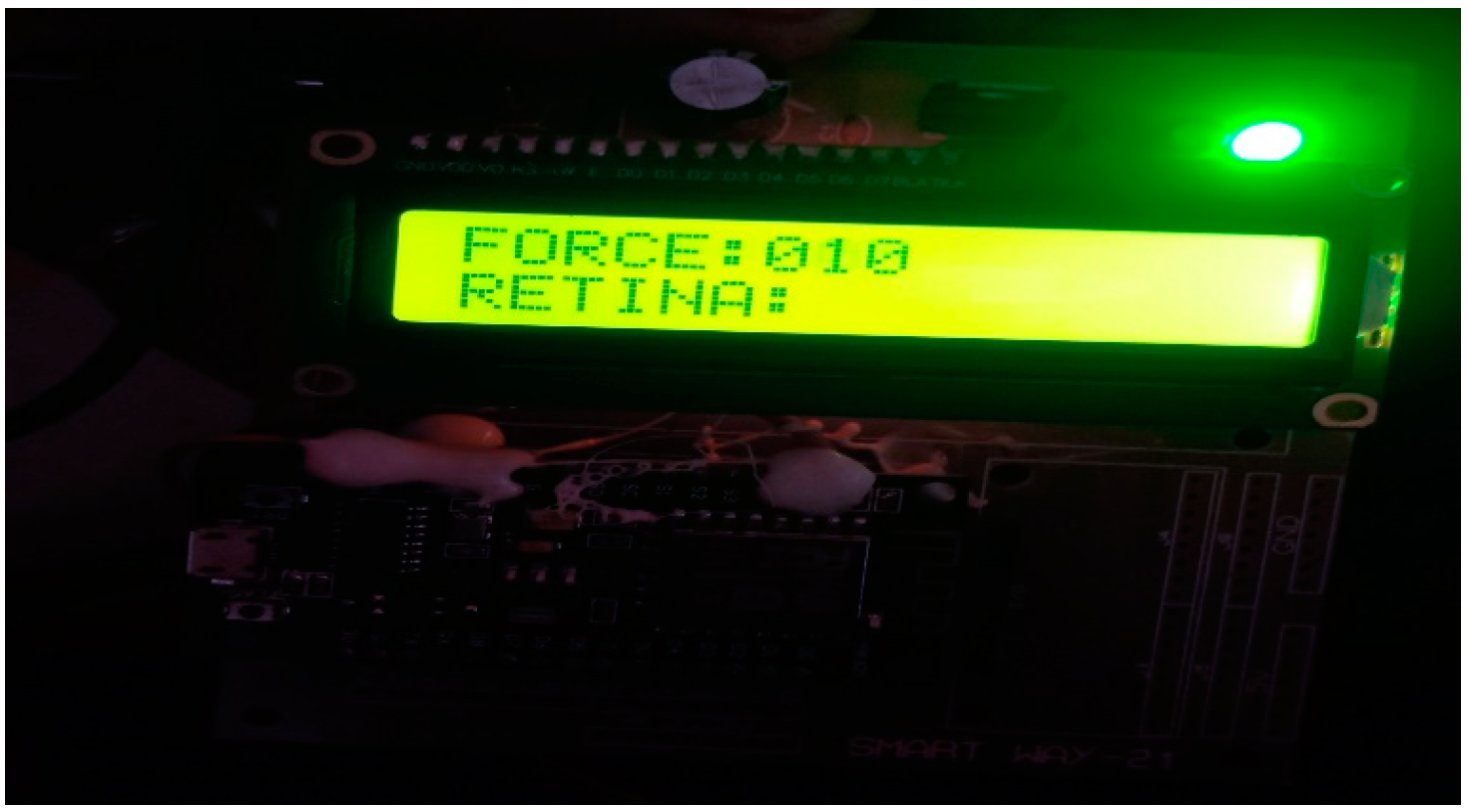
- Output Module: The output module is responsible for presenting the results or information derived from the measurement of intraocular pressure. This can be in the form of visual displays, audio signals, or data transmission to external devices or systems. The output module enables healthcare professionals or users to interpret and utilize the measured intraocular pressure data effectively.
3.2. Microcontroller
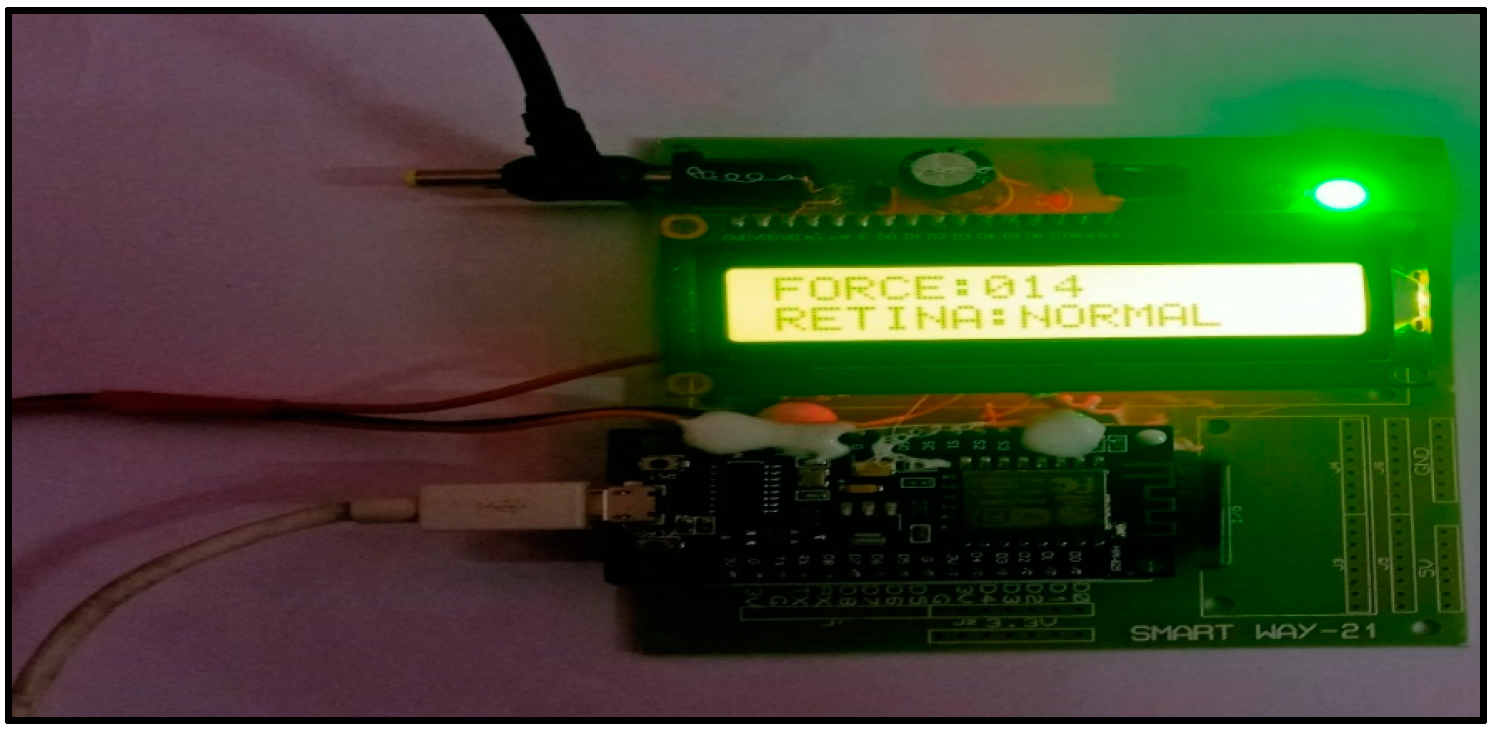
3.2.1. NODE MCU ESP8266
3.2.2. Force Sensor
3.2.3. Principle: Fingertip-Test Method
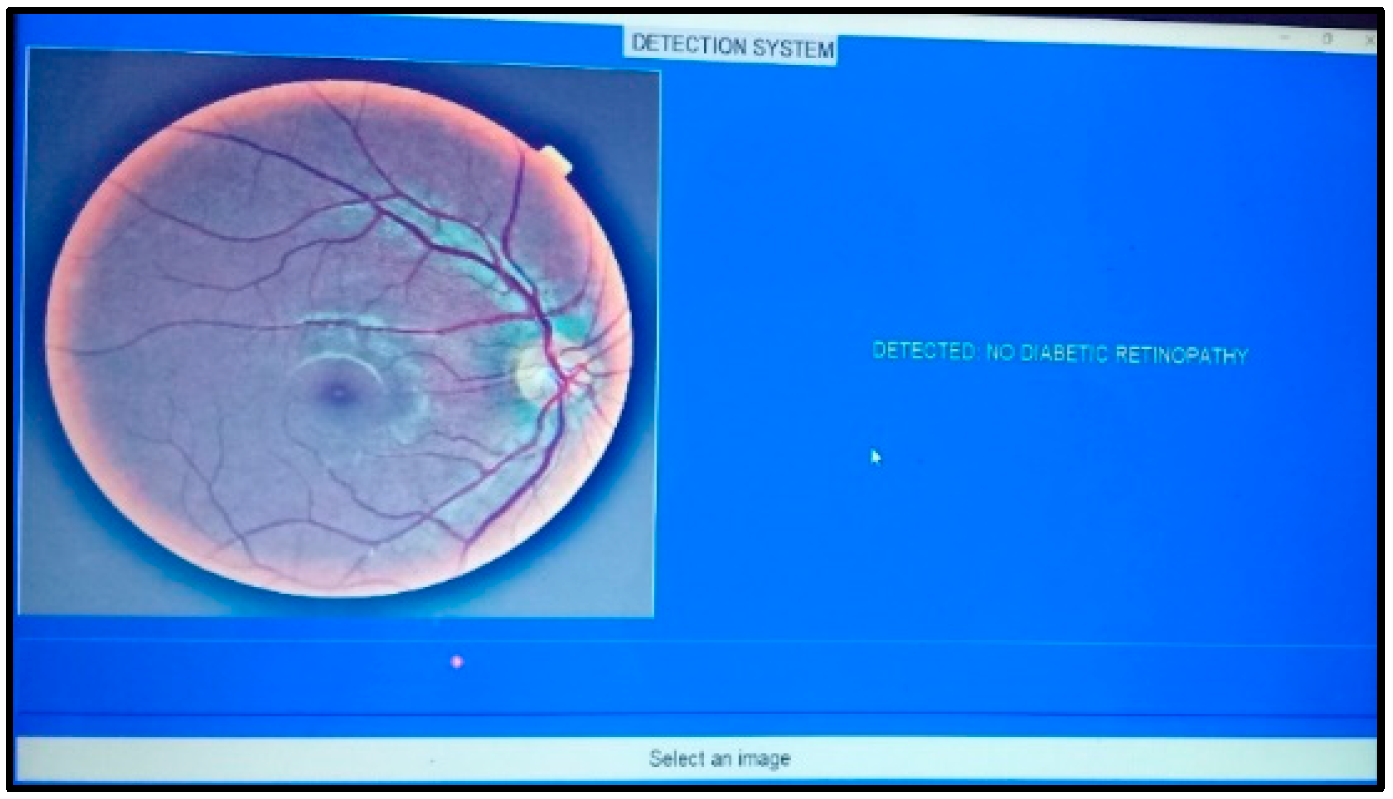
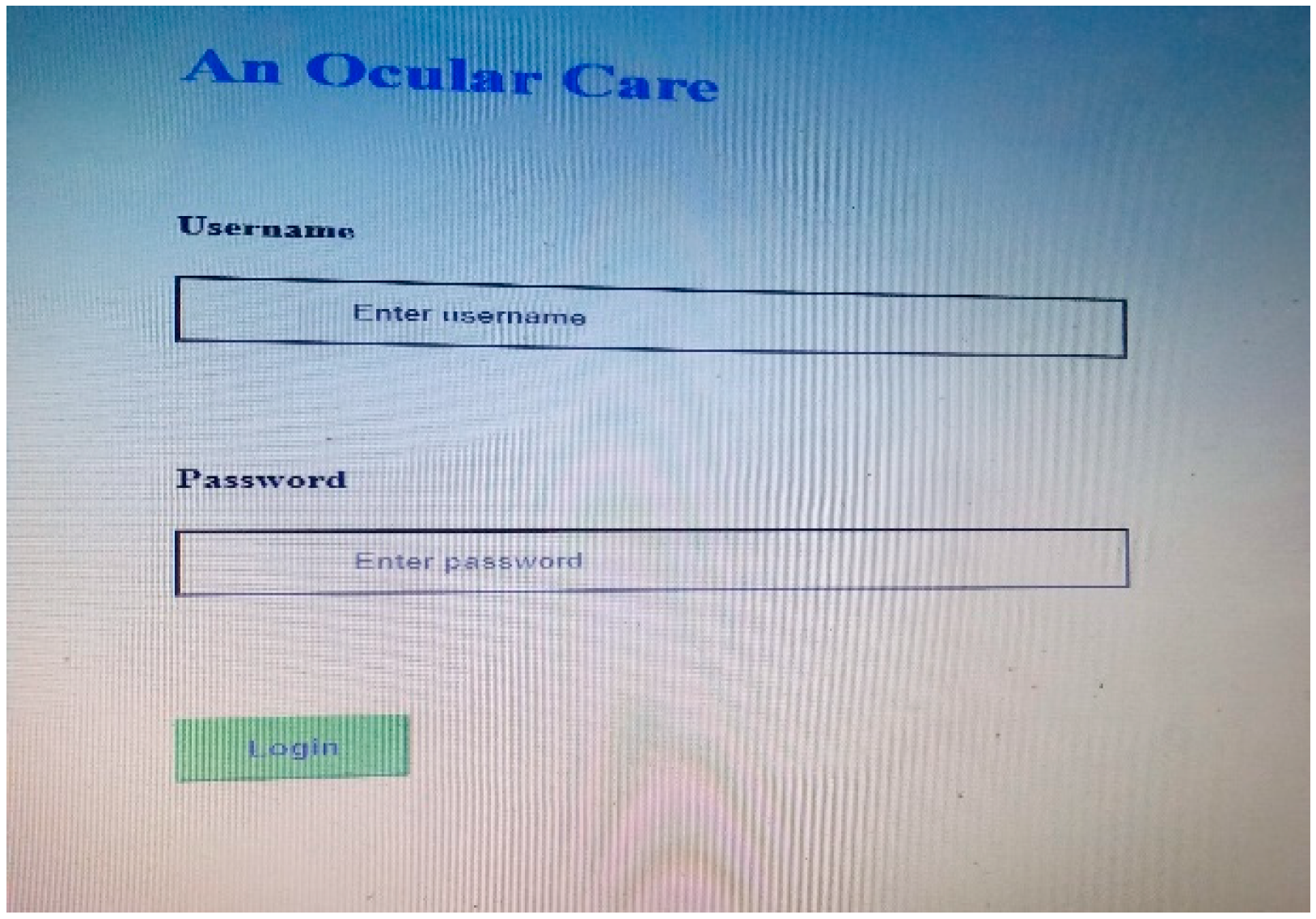
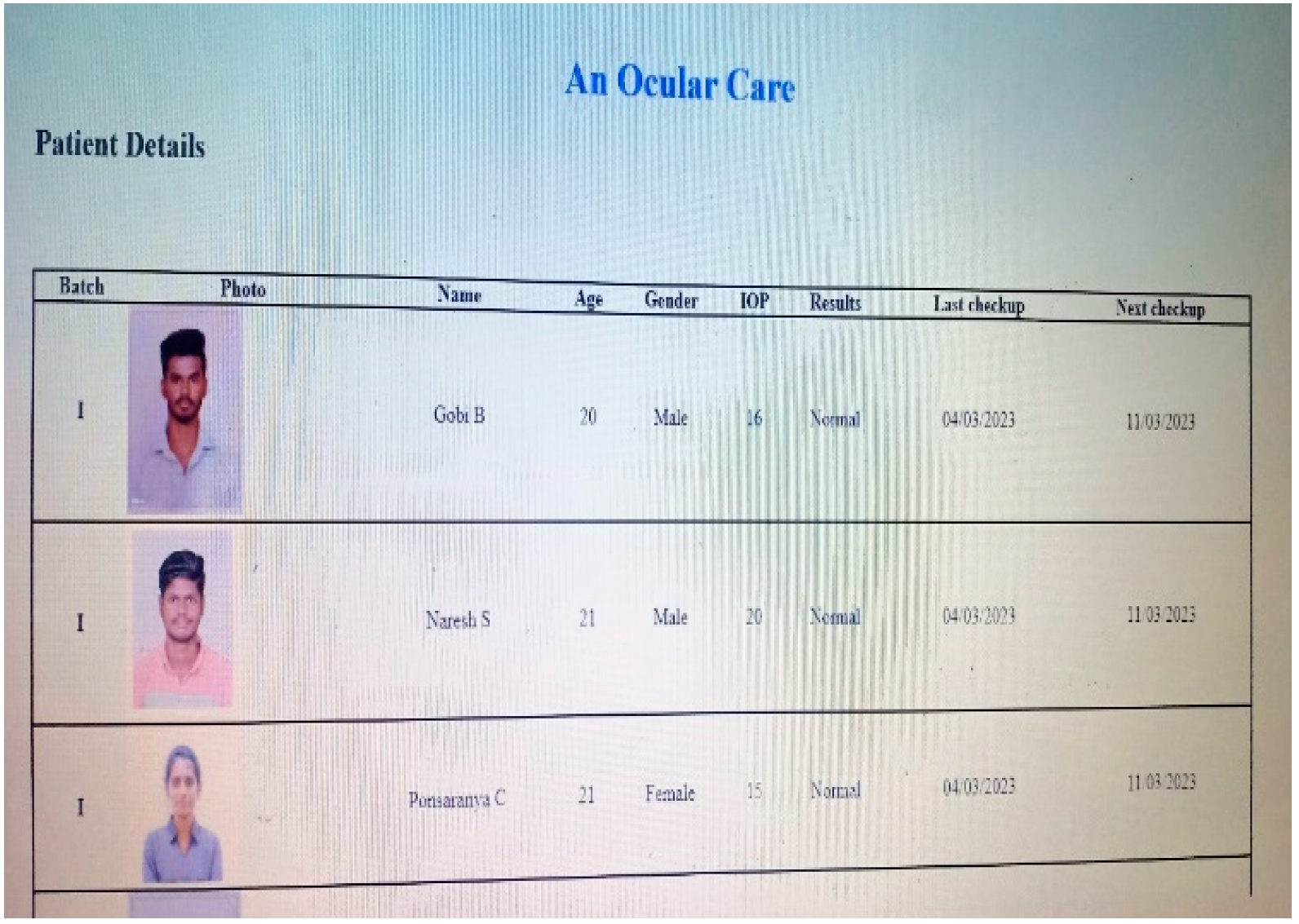
4. Results and Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agaoglu, S.; Diep, P.; Martini, M.; Kt, S.; Baday, M.; Araci, I.E. Ultra-sensitive microfluidic wearable strainsensor for intraocular pressure monitoring. Lab A Chip 2018, 18, 3471–3483. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Chen, L.; Liu, X.; Zhao, B.; Zhang, H.; Wu, Z. Microfluidic contact lenses for unpowered, continuous and non-invasive intraocular pressure monitoring. Sens. Actuators A Phys. 2019, 295, 177–187. [Google Scholar]
- Brezhnev, A.Y.; Baranov, V.I.; Kuroyedov, A.V.; Petrov, S.Y.; Antonov, A.A. 24-hour intraocular pressure monitoring: Opportunities and challenges. Natl. J. Glaucoma 2018, 17, 77–85. [Google Scholar]
- Craig, J.E.; Han, X.; Qassim, A.; Hassall, M.; Cooke Bailey, J.N.; Kinzy, T.G.; Khawaja, A.P.; An, J.; Marshall, H.; Gharahkhani, P.; et al. Multitrait analysis of glaucoma identifies new risk loci and enables polygenic prediction of disease susceptibility and progression. Nat. Genet. 2020, 52, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Cvenkel, B.; Atanasovska Velkovska, M. Self-monitoring of intraocular pressure using Icare HOME tonometry in clinical practice. Clin. Ophthalmol. 2019, 13, 841–847. [Google Scholar] [CrossRef]
- Devalla, S.K.; Liang, Z.; Pham, T.H.; Boote, C.; Strouthidis, N.G.; Thiery, A.H.; Girard, M.J. Glaucoma management in the era of artificial intelligence. Br. J. Ophthalmol. 2020, 104, 301–311. [Google Scholar] [PubMed]
- Enders, P.; Hall, J.; Bornhauser, M.; Mansouri, K.; Altay, L.; Schrader, S.; Dietlein, T.S.; Bachmann, B.O.; Neuhann, T.; Cursiefen, C. Telemetric intraocular pressure monitoring after boston keratoprosthesis surgery using the eyemate-IO sensor: Dynamics in the first year. Am. J. Ophthalmol. 2019, 206, 256–263. [Google Scholar] [CrossRef]
- Garg, A.; Vickerstaff, V.; Nathwani, N.; Garway-Heath, D.; Konstantakopoulou, E.; Ambler, G.; Bunce, C.; Wormald, R.; Barton, K.; Gazzard, G.; et al. Efficacy of repeat selective laser trabeculoplasty in medication-naive open-angle glaucoma and ocular hypertension during the LiGHT trial. Ophthalmology 2020, 127, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Karunaratne, I.K.; Lee CH, C.; Or, P.W.; Wei, Y.; Chong, I.T.; Yang, Y.; Yu, M.; Lam, D.C.C. Wearable dual-element intraocular pressure contact lens sensor. Sens. Actuators A Phys. 2021, 321, 112580. [Google Scholar] [CrossRef]
- Kim, J.; Park, J.; Park, Y.G.; Cha, E.; Ku, M.; An, H.S.; Lee, K.-P.; Huh, M.-I.; Kim, J.; Kim, T.-S.; et al. A soft and transparent contact lens for the wireless quantitative monitoring of intraocular pressure. Nat. Biomed. Eng. 2021, 5, 772–782. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, J.; Ku, M.; Cha, E.; Ju, S.; Park, W.Y.; Kim, K.H.; Kim, D.W.; Berggren, P.-O.; Park, J.U. Intraocular pressure monitoring following islet transplantation to the anterior chamber of the eye. Nano Lett. 2019, 20, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Tavakoli, A.; Weber, A.J.; Li, W. Wireless, passive strain sensor in a doughnut-shaped contact lens for continuous non-invasive self-monitoring of intraocular pressure. Lab A Chip 2020, 20, 332–342. [Google Scholar]
- Yadav, K.S.; Rajpurohit, R.; Sharma, S. Glaucoma: Current treatment and impact of advanced drug delivery systems. Life Sci. 2019, 221, 362–376. [Google Scholar] [PubMed]
- Stein, J.D.; Khawaja, A.P.; Weizer, J.S. Glaucoma in adults-screening, diagnosis, and management. JAMA 2021, 325, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xu, M.; Liu, H.; Li, Y.; Wang, X.; Jiang, L.; Wamg, Z.; Fan, X.; Wang, N. A large-scale database and a CNN model for attention-based glaucoma detection. IEEE Trans. Med. Imaging 2019, 39, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xu, M.; Wang, X.; Jiang, L.; Liu, H. Attention based glaucoma detection: A large-scale database and CNN model. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition, Long Beach, CA, USA, 16–20 June 2019; pp. 10571–10580. [Google Scholar]
- Pang, Y.; Li, Y.; Wang, X.; Qi, C.; Yang, Y.; Ren, T.-L. A contact lens promising for non-invasive continuous intraocular pressure monitoring. RSC Adv. 2019, 9, 5076–5082. [Google Scholar] [CrossRef] [PubMed]
- Rathi, S.; Andrews, C.A.; Greenfield, D.S.; Stein, J.D. Trends in glaucoma surgeries performed by glaucoma subspecialists versus nmb. Ophthalmology 2021, 128, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, I.; Martin, R. Advances in diagnostic applications for monitoring intraocular pressure in Glaucoma: A review. J. Optom. 2019, 12, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Saxby, E.; Mansouri, K.; Tatham, A.J. Intraocular pressure monitoring using an intraocular sensor before and after glaucoma surgery. J. Glaucoma 2021, 30, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.Y.; Jung, K.I.; Park, H.Y.L.; Park, C.K. The effect of anxiety and depression on progression of glaucoma. Sci. Rep. 2021, 11, 1769. [Google Scholar] [PubMed]






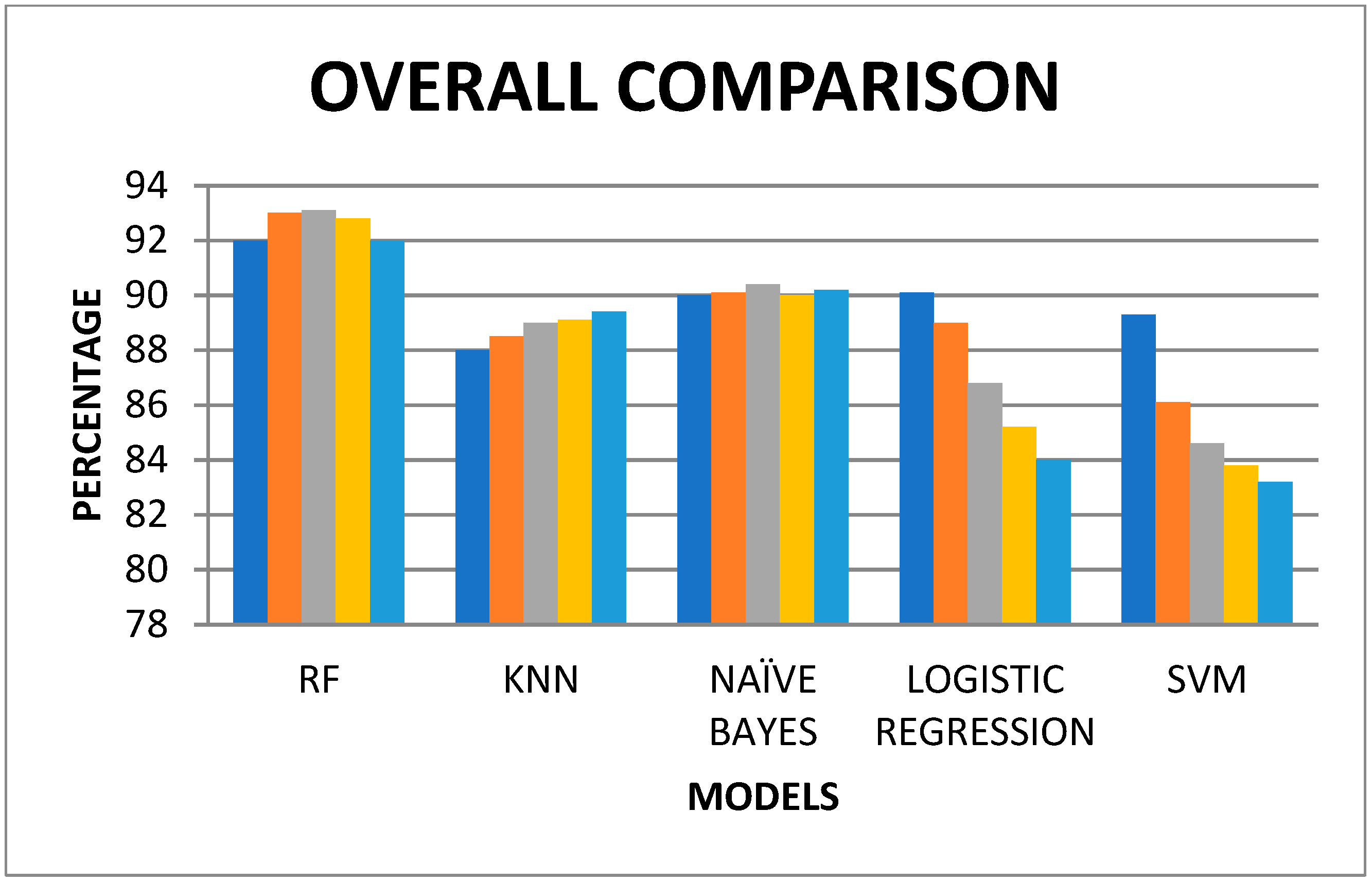
| Training & Testing | RF | KNN | NAÏVE BAYES | LOGISTIC REGRESSION | SVM |
|---|---|---|---|---|---|
| 50 & 50 | 92 | 88 | 90 | 90.1 | 89.3 |
| 60 & 40 | 93 | 88.5 | 90.1 | 89 | 86.1 |
| 70 & 30 | 93.1 | 89 | 90.4 | 86.8 | 84.6 |
| 80 & 20 | 92.8 | 89.1 | 90 | 85.2 | 83.8 |
| 90 & 10 | 92 | 89.4 | 90.2 | 84 | 83.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chinnaswamy, S.; Natarajan, V.; Samiappan, S.; Gurumurthy, R. Intraocular Pressure Monitoring System for Glaucoma Patients Using IoT and Machine Learning. Eng. Proc. 2023, 59, 179. https://doi.org/10.3390/engproc2023059179
Chinnaswamy S, Natarajan V, Samiappan S, Gurumurthy R. Intraocular Pressure Monitoring System for Glaucoma Patients Using IoT and Machine Learning. Engineering Proceedings. 2023; 59(1):179. https://doi.org/10.3390/engproc2023059179
Chicago/Turabian StyleChinnaswamy, Sivamani, Vigneshwari Natarajan, Selvi Samiappan, and Revathy Gurumurthy. 2023. "Intraocular Pressure Monitoring System for Glaucoma Patients Using IoT and Machine Learning" Engineering Proceedings 59, no. 1: 179. https://doi.org/10.3390/engproc2023059179
APA StyleChinnaswamy, S., Natarajan, V., Samiappan, S., & Gurumurthy, R. (2023). Intraocular Pressure Monitoring System for Glaucoma Patients Using IoT and Machine Learning. Engineering Proceedings, 59(1), 179. https://doi.org/10.3390/engproc2023059179






