Abstract
Autism spectrum disorder (ASD) is a global concern, with a prevalence rate of approximately 1 in 36 children according to estimates from the Centers for Disease Control and Prevention (CDC). Diagnosing ASD poses challenges due to the absence of a definitive medical test. Instead, doctors rely on a comprehensive evaluation of a child’s developmental background and behavior to reach a diagnosis. Although ASD can occasionally be identified in children aged 18 months or younger, a reliable diagnosis by an experienced professional is typically made by the age of two. Early detection of ASD is crucial for timely interventions and improved outcomes. In recent years, the field of early diagnosis of ASD has been greatly impacted by the emergence of deep learning models, which have brought about a revolution by greatly improving the accuracy and efficiency of ASD detection. The objective of this review paper is to examine the recent progress in early ASD detection through the utilization of multimodal deep learning techniques. The analysis revealed that integrating multiple modalities, including neuroimaging, genetics, and behavioral data, is key to achieving higher accuracy in early ASD detection. It is also evident that, while neuroimaging data holds promise and has the potential to contribute to higher accuracy in ASD detection, it is most effective when combined with other modalities. Deep learning models, with their ability to analyze complex patterns and extract meaningful features from large datasets, offer great promise in addressing the challenge of early ASD detection. Among various models used, CNN, DNN, GCN, and hybrid models have exhibited encouraging outcomes in the early detection of ASD. The review highlights the significance of developing accurate and easily accessible tools that utilize artificial intelligence (AI) to aid healthcare professionals, parents, and caregivers in early ASD symptom recognition. These tools would enable timely interventions, ensuring that necessary actions are taken during the initial stages.
1. Introduction
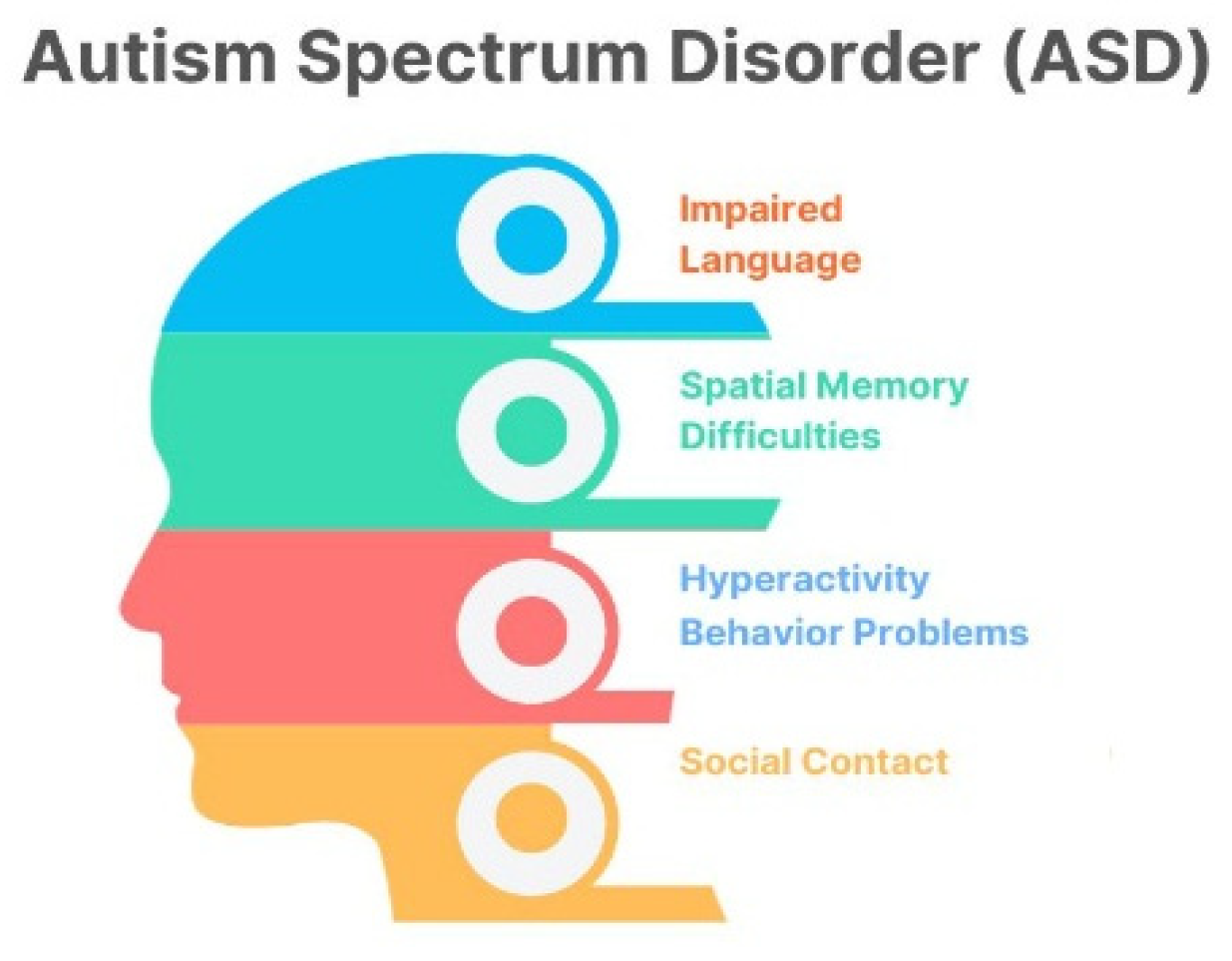
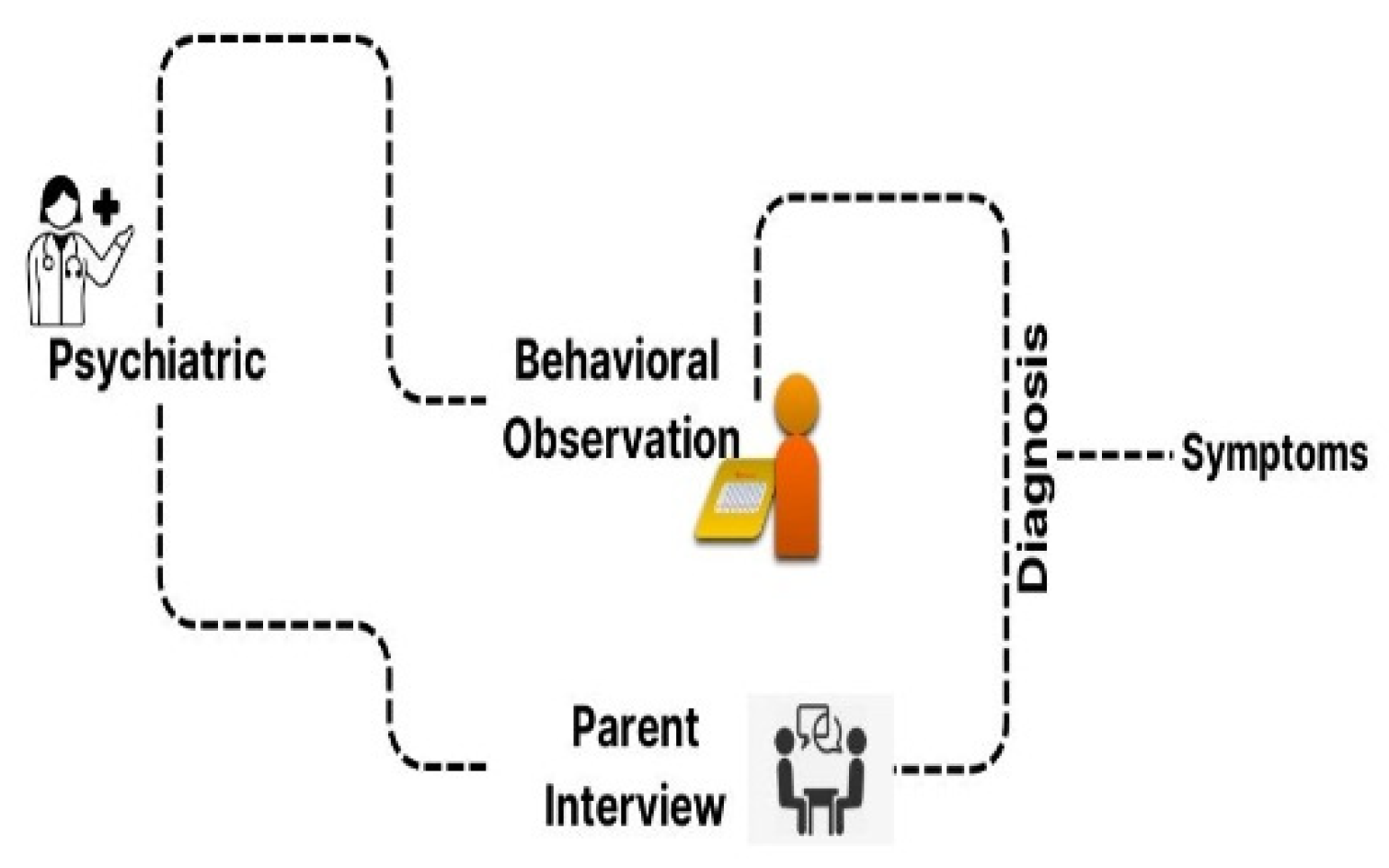
Autism spectrum disorder (ASD) is a developmental condition affecting 1–2% of children worldwide, causing social interaction challenges, communication difficulties, and repetitive behaviors. Figure 1 shows the issues faced by children with ASD. Genetics and environmental factors significantly impact its development. Advances in diagnosis provide hope for improved outcomes [1,2,3,4]. ASD individuals face challenges such as social interaction difficulties, communication issues, repetitive behaviors, and sensory sensitivities [5,6,7,8]. The assessment and diagnosis of ASD largely rely on traditional clinical evaluations that have been utilized for several decades, as shown in Figure 2. Deep learning techniques are increasingly used for ASD detection, and integrate data from various sources to enhance accuracy [9]. The choice of modalities depends on available data and research goals [10]. Deep learning (DL) methods are increasingly used in early ASD detection and for analyzing data from neuroimaging, behavioral observations, and speech [11]. This enhances diagnostic accuracy and timeliness, potentially improving outcomes [12]. fMRI and sMRI play vital roles in accurate diagnosis [13]. AI-based CAS employs both ML and DL approaches, but DL techniques are underutilized [14,15,16]. Advancements in ASD diagnostics use DL models, combining neuroimaging methods with ML and DL, to identify early biological markers [17,18,19]. Lightweight CNN models show high accuracy, precision, and F1 score. Challenges include data quality, interpretability, generalizability, and ethical considerations [14,20].
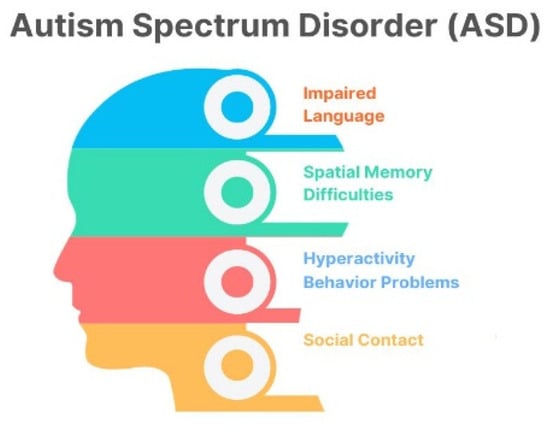
Figure 1.
Behavioral issues in ASD children.
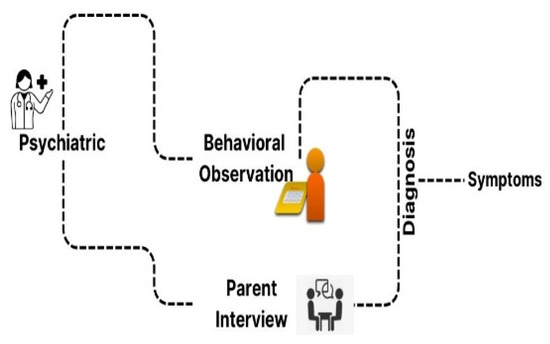
Figure 2.
Traditional clinical evaluation for ASD.
2. Methodology
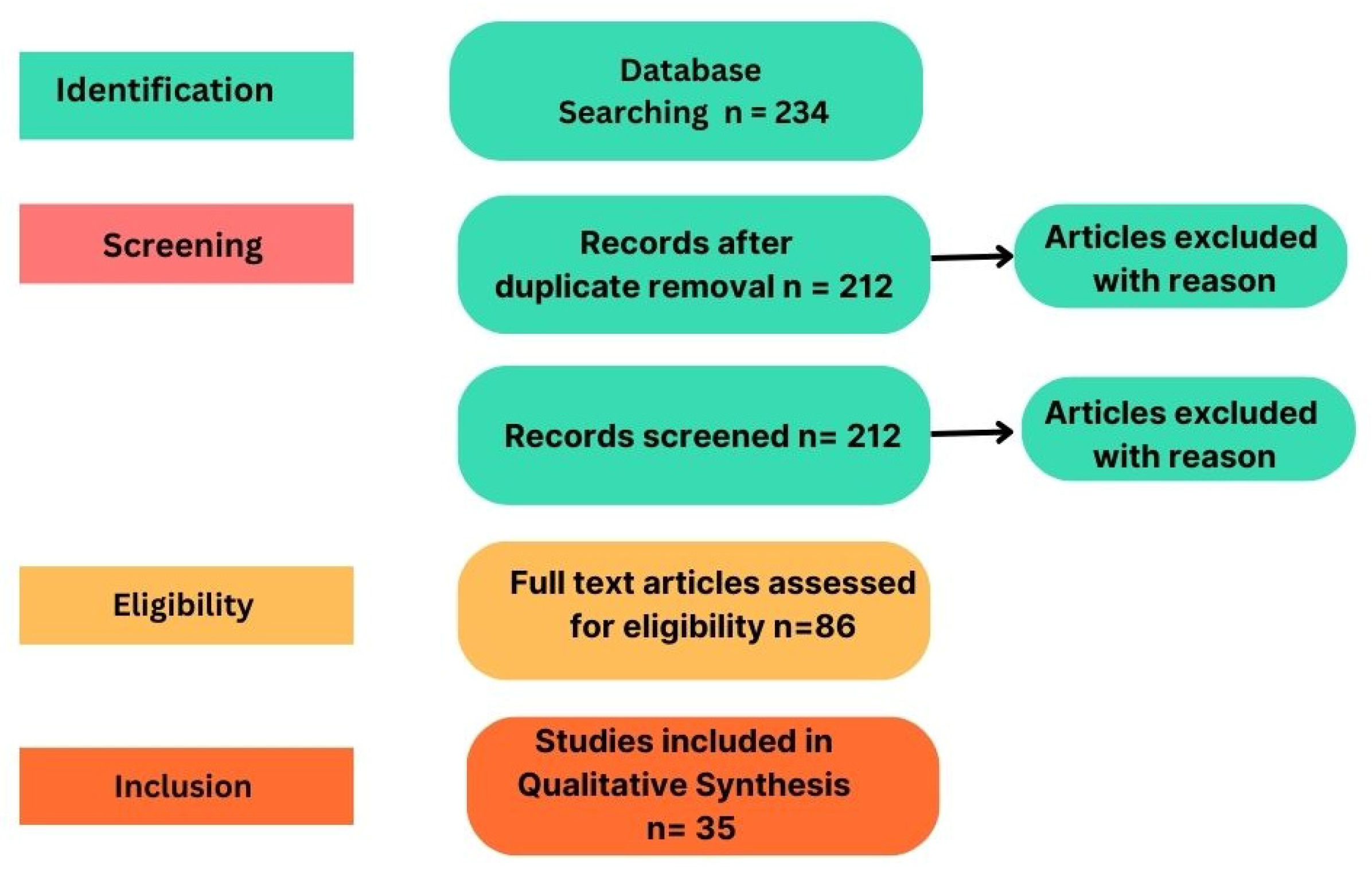
This systematic review uses PRISMA methodology to analyze early ASD detection advancements using multimodal DL techniques. It employs structured research methods, including clear questions, eligibility criteria, literature search, systematic screening, and data extraction. The review discusses implications and challenges, considering strengths and limitations. A systematic search approach was used to evaluate each article’s suitability to address the research questions. In this review, databases like Google Scholar, PubMed, and IEEE were used to acquire the current study of neurodevelopmental disorders in children using machine learning techniques. Relevant articles were shortlisted using keywords like “Deep Learning” and “Autism Spectrum Disorder”. Figure 3 shows the flow and the number of articles identified through different sources, which focused on publications from 2019–2023. After thorough examination of titles, abstracts, and full contents, 35 articles were selected for further analysis.
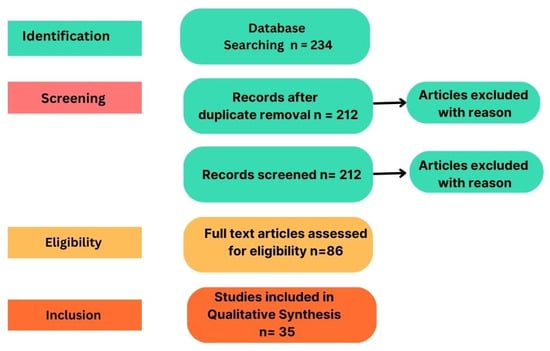
Figure 3.
PRISMA review methodology.
3. Data Synthesis and Analysis
Several studies have delved into recent advancements in early ASD detection through multimodal DL techniques using neuroimaging and non-neuroimaging data. Khodatars et al. [9] explored DL and AI’s role in precise ASD diagnosis and rehabilitation, offering insights for future research directions. de Belen et al. [21] highlighted the effectiveness of computer vision analysis in quantifying ASD markers, benefiting diagnosis and therapy. Feng et al. [22] assessed ML and DL methods for fMRI-based ASD classification and recognition, discussing performance and challenges in early diagnosis. Haweel et al. [23] investigated the potential of deep learning techniques using TfMRI for early ASD diagnosis and the identification of autism biomarkers. Table 1 shows a summary of multimodal DL techniques using neuroimaging and non-neuroimaging techniques in ASD detection.

Table 1.
Summary of multimodal DL techniques in ASD detection as reported in previous studies.
4. Modalities Used in ASD Detection
In the detection of autism spectrum disorder (ASD), a combination of neuroimaging and non-neuroimaging techniques is employed to assess various aspects of an individual’s behavior, cognition and neurological function. Table 2 gives an overview of both types of modalities used to detect autism spectrum disorder at an early stage.

Table 2.
Various deep learning ASD detection modalities using neuroimaging and non-neuroimaging techniques and their description.
5. Deep Learning Models
Various neural network models are pivotal in improving ASD detection. CNNs excel at tasks like facial analysis, eye-tracking, and speech analysis, enhancing diagnostic accuracy and enabling personalized interventions [30,31,32]. DNNs are proficient at extracting complex patterns, aiding early detection, diagnosis, and personalized interventions [37,41,44]. RNNs are instrumental when analyzing sequential data and speech transcripts, supporting early screening and personalized interventions [24]. GCNs contribute by capturing relationships in neuroimaging and social interaction graphs, improving diagnostic accuracy and advancing ASD research [27,39,52]. These neural network models collectively enhance ASD detection across diverse data modalities.
6. Performance Analysis
The analysis shown in Table 1 presents the accuracy results from various ASD classification models, demonstrating advancements in deep learning for ASD detection. High-performing models include ASDL (98.2%), EfficientNetB1 (95.06%), CNN (94%), and MobileNet (95%). Competitively performing models include CNN (87.50%) and GCN (79.5%), while BLSTM (68.18%) and Graph-based multi-model ensemble (73.13%) achieve lower accuracies. This underscores the importance of choosing appropriate deep learning architectures for accurate ASD classification, offering insights for future research and clinical applications.
7. Research Gaps and Future Directions
The literature review identified several limitations in ASD detection, including limited multimodal data-based studies, lack of longitudinal studies, lack of explainability and interpretability, limited data size, and overall limitations. These issues require future research to address neuroimaging, genetic information, and behavioral assessments for improved accuracy and reliability. Addressing these issues is crucial for enhancing effectiveness and reliability in diverse datasets and populations.
Multimodal data integration in ASD detection faces challenges in feature integration, interpretability, and data consistency. Robust fusion techniques are needed for resource-intensive data collection. Collaboration with clinicians is crucial for practical effectiveness. Online learning and adaptive models are essential. Longitudinal analysis is crucial for personalized treatment plans. Innovations in DL models improve prediction accuracy and treatment strategies.
8. Conclusions
This review highlights recent advancements in early ASD detection using multimodal deep learning techniques, enhancing accuracy and objectivity. These techniques integrate behavioral, genetic, and neuroimaging data, enabling personalized interventions and standardized assessment processes. However, further research is needed to address challenges like improved detection accuracy, data availability and interpretability. Multimodal deep learning techniques for early ASD detection offer significant scientific implications, improving accuracy and reducing diagnostic inconsistencies. By integrating behavioral, genetic, and neuroimaging data, these techniques enable standardized assessments, personalized interventions, and large-scale screening. However, further research and validation are needed before widespread implementation in clinical settings.
Author Contributions
All authors discussed the contents of the manuscript. S.S.D. contributed to the research idea, database searching, filtering of papers and comparison study with conclusion, and writing; J.P. reviewed and suggested changes to the manuscript and to the flow of study to be followed. All authors have read and agreed to the published version of the manuscript.
Funding
The research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors would like to thank the reviewers for the constructive comments, which improved overall quality of this paper.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ASSDL | Attention based semi-supervised dictionary learning |
| DBN | Deep belief network |
| GCN | Graph convolutional networks |
| ROI | Regions of interest |
| AAL | Anatomical automatic labeling |
| rs-fMRI | Resting-state fMRI |
| PC | Personal characteristic |
| MISO-DNN | Multi-input single-output deep neural network |
| ADOS-2 | Autism diagnostic observation schedule, second edition |
| BLSTM | Bidirectional long short-term memory |
| ADI-R | Autism diagnostic interview, revised |
| BeDevel-I | Behavior development screening for toddlers interview |
| BeDevel-P | Behavior development screening for toddlers play |
| K-CARS | Korean version of the childhood autism rating scale |
| SCQ | Social communication questionnaire |
| SRS | Social responsiveness scale |
| QCHAT | Quantitative checklist for autism in toddlers |
| DANN | Multichannel deep attention neural network |
| DNN | Deep neural network |
| DEAF | Deep extreme adaptive fuzzy |
| RAPID | Real-time analysis of precursors for intervention and detection |
| MTFS | Multi-task feature selection |
| eGeMAPS | Geneva minimalistic acoustic parameter set |
| MMSDAE | Multimodal stacked denoising autoencoder |
| DFC | Dynamic functional connectivity |
| SC-CNN | Separated channel convolutional neural network |
| CAE | Convolutional autoencoder |
| RSFC | Resting-state functional connectivity |
| DeepMNF | Deep multimodal neuroimaging framework |
| maLRR | multi-site adaption framework via low-rank representation |
| AAL | Anatomical automatic labeling |
| PRISMA | Preferred reporting items for systematic reviews and meta-analyses |
| ABIDE | Autism brain imaging data exchange |
References
- Bjørklund, G.; Pivina, L.; Dadar, M.; Meguid, N.A.; Semenova, Y.; Anwar, M.; Chirumbolo, S. Gastrointestinal alterations in autism spectrum disorder: What do we know? Neurosci. Biobehav. Rev. 2020, 118, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.; Fewster, D.L.; Gurayah, T. Parents’ voices: Experiences and coping as a parent of a child with autism spectrum disorder. S. Afr. J. Occup. Ther. 2019, 49, 43–50. [Google Scholar] [CrossRef]
- Wiśniowiecka-Kowalnik, B.; Nowakowska, B.A. Genetics and epigenetics of autism spectrum disorder—Current evidence in the field. J. Appl. Genet. 2019, 60, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Schaaf, C.P.; Betancur, C.; Yuen, R.K.; Parr, J.R.; Skuse, D.H.; Gallagher, L.; Bernier, R.A.; Buchanan, J.A.; Buxbaum, J.D.; Chen, C.A.; et al. A framework for an evidence-based gene list relevant to autism spectrum disorder. Nat. Rev. Genet. 2020, 21, 367–376. [Google Scholar] [CrossRef]
- Kong, C.; Chen, A.; Ludyga, S.; Herold, F.; Healy, S.; Zhao, M.; Taylor, A.; Müller, N.G.; Kramer, A.F.; Chen, S.; et al. Associations between meeting 24-hour movement guidelines and quality of life among children and adolescents with autism spectrum disorder. J. Sport Health Sci. 2023, 12, 73–86. [Google Scholar] [CrossRef]
- Tomczak, M.T. How can the work environment be redesigned to enhance the well-being of individuals with autism? Empl. Relat. Int. J. 2022, 44, 1467–1484. [Google Scholar] [CrossRef]
- Singh, D.; Rakhra, M.; Aggarwal, S. Autism Spectrum Disorder Detection using theDeep Learning Approaches. In Proceedings of the 2022 2nd International Conference on Technological Advancements in Computational Sciences (ICTACS), Tashkent, Uzbekistan, 10–12 October 2022; pp. 761–766. [Google Scholar]
- Yen, C.; Chiang, M.C. Examining the effect of online advertisement cues on human responses using eye-tracking, EEG, and MRI. Behav. Brain Res. 2021, 402, 113128. [Google Scholar] [CrossRef]
- Khodatars, M.; Shoeibi, A.; Sadeghi, D.; Ghaasemi, N.; Jafari, M.; Moridian, P.; Khadem, A.; Alizadehsani, R.; Zare, A.; Kong, Y.; et al. Deep learning for neuroimaging-based diagnosis and rehabilitation of autism spectrum disorder: A review. Comput. Biol. Med. 2021, 139, 104949. [Google Scholar] [CrossRef]
- Albahri, A.S.; Al-Qaysi, Z.T.; Alzubaidi, L.; Alnoor, A.; Albahri, O.S.; Alamoodi, A.H.; Bakar, A.A. A systematic review of using deep learning technology in the steady-state visually evoked potential-based brain-computer interface applications: Current trends and future trust methodology. Int. J. Telemed. Appl. 2023, 2023, 7741735. [Google Scholar] [CrossRef]
- Zwaigenbaum, L.; Brian, J.A.; Ip, A. Early detection for autism spectrum disorder in young children. Paediatr. Child Health 2019, 24, 424–432. [Google Scholar] [CrossRef]
- McCarty, P.; Frye, R.E. Early detection and diagnosis of autism spectrum disorder: Why is it so difficult? Semin. Pediatr. Neurol. 2020, 35, 100831. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Li, X.; Hu, L.; Lu, L. Integrating genomic and resting State fMRI for efficient autism spectrum disorder classification. Multimed. Tools Appl. 2022, 81, 19183–19194. [Google Scholar] [CrossRef]
- Moridian, P.; Ghassemi, N.; Jafari, M.; Salloum-Asfar, S.; Sadeghi, D.; Khodatars, M.; Shoeibi, A.; Khosravi, A.; Ling, S.H.; Subasi, A.; et al. Automatic autism spectrum disorder detection using artificial intelligence methods with MRI neuroimaging: A review. Front. Mol. Neurosci. 2022, 15, 999605. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.L.; Wang, S.H.; Liu, W.B.; Zhu, H.L.; Li, M.; Zou, X.B. A multimodal machine learning system in early screening for toddlers with autism spectrum disorders based on the response to name. Front. Psychiatry 2023, 14, 1039293. [Google Scholar] [CrossRef]
- Mohanty, A.S.; Parida, P.; Patra, K.C. Usage of ML Techniques for ASD Detection: A Comparative Analysis of Various Classifiers. In Machine Learning and Deep Learning in Medical Data Analytics and Healthcare Applications; CRC Press: Boca Raton, FL, USA, 2022; pp. 91–112. [Google Scholar]
- Kaur, P.; Kaur, A. Review of progress in diagnostic studies of autism spectrum disorder using neuroimaging. Interdiscip. Sci. Comput. Life Sci. 2023, 15, 111–130. [Google Scholar] [CrossRef]
- Kohli, M.; Kar, A.K.; Sinha, S. The role of intelligent technologies in early detection of autism spectrum disorder (asd): A scoping review. IEEE Access 2022, 10, 104887–104913. [Google Scholar] [CrossRef]
- Dawson, G.; Bernier, R. A quarter century of progress on the early detection and treatment of autism spectrum disorder. Dev. Psychopathol. 2013, 25, 1455–1472. [Google Scholar] [CrossRef]
- Sundas, A.; Badotra, S.; Rani, S.; Gyaang, R. Evaluation of autism spectrum disorder based on the healthcare by using artificial intelligence strategies. J. Sens. 2023, 2023, 5382375. [Google Scholar] [CrossRef]
- de Belen RA, J.; Bednarz, T.; Sowmya, A.; Del Favero, D. Computer vision in autism spectrum disorder research: A systematic review of published studies from 2009 to 2019. Transl. Psychiatry 2020, 10, 333. [Google Scholar] [CrossRef]
- Feng, W.; Liu, G.; Zeng, K.; Zeng, M.; Liu, Y. A review of methods for classification and recognition of ASD using fMRI data. J. Neurosci. Methods 2022, 368, 109456. [Google Scholar] [CrossRef]
- Haweel, R.; Shalaby, A.; Mahmoud, A.; Seada, N.; Ghoniemy, S.; Ghazal, M.; Casanova, M.F.; Barnes, G.N.; El Baz, A. A robust DWT–CNN-based CAD system for early diagnosis of autism using task-based fMRI. Med. Phys. 2021, 48, 2315–2326. [Google Scholar] [CrossRef]
- Li Ming Tang, D.; Zeng, J.; Zhou, T.; Zhu, H.; Chen, B.; Zou, X. An automated assessment framework for atypical prosody and stereotyped idiosyncratic phrases related to autism spectrum disorder. Comput. Speech Lang. 2019, 56, 80–94. [Google Scholar]
- Yang, M.; Zhong, Q.; Chen, L.; Huang, F.; Lei, B. Attention based semi-supervised dictionary learning for diagnosis of autism spectrum disorders. In Proceedings of the 2019 IEEE International Conference on Multimedia & Expo Workshops (ICMEW), Shanghai, China, 8–12 July 2019; pp. 7–12. [Google Scholar]
- Huang, Z.A.; Zhu, Z.; Yau, C.H.; Tan, K.C. Identifying autism spectrum disorder from resting-state fMRI using deep belief network. IEEE Trans. Neural Netw. Learn. Syst. 2020, 32, 2847–2861. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Liu, J.; Shi, M.; Wong, C.W.; Chan KH, K. Identifying autism spectrum disorder based on individual-aware down-sampling and multi-modal learning. arXiv 2021, arXiv:2109.09129. [Google Scholar]
- Niu, K.; Guo, J.; Pan, Y.; Gao, X.; Peng, X.; Li, N.; Li, H. Multichannel deep attention neural networks for the classification of autism spectrum disorder using neuroimaging and personal characteristic data. Complexity 2020, 2020, 1357853. [Google Scholar] [CrossRef]
- Ahmed, Z.A.; Aldhyani, T.H.; Jadhav, M.E.; Alzahrani, M.Y.; Alzahrani, M.E.; Althobaiti, M.M.; Alassery, F.; Alshaflut, A.; Alzahrani, N.M.; Al-Madani, A.M. Facial features detection system to identify children with autism spectrum disorder: Deep learning models. Comput. Math. Methods Med. 2022, 2022, 3941049. [Google Scholar] [CrossRef]
- Saputra DC, E.; Maulana, Y.; Win, T.A.; Phann, R.; Caesarendra, W. Implementation of Machine Learning and Deep Learning Models Based on Structural MRI for Identification of Autism Spectrum Disorder. JurnalIlmiah Tek. ElektroKomputer Dan Inform. (JITEKI) 2023, 9, 307–318. [Google Scholar]
- Liao, M.; Duan, H.; Wang, G. Application of machine learning techniques to detect the children with autism spectrum disorder. J. Healthc. Eng. 2022, 2022, 9340027. [Google Scholar] [CrossRef]
- Sharif, H.; Khan, R.A. A novel machine learning based framework for detection of autism spectrum disorder (ASD). Appl. Artif. Intell. 2022, 36, 2004655. [Google Scholar] [CrossRef]
- Epalle, T.M.; Song, Y.; Liu, Z.; Lu, H. Multi-atlas classification of autism spectrum disorder with hinge loss trained deep architectures: ABIDE I results. Appl. Soft Comput. 2021, 107, 107375. [Google Scholar] [CrossRef]
- Ke, F.; Choi, S.; Kang, Y.H.; Cheon, K.A.; Lee, S.W. Exploring the structural and strategic bases of autism spectrum disorders with deep learning. IEEE Access 2020, 8, 153341–153352. [Google Scholar] [CrossRef]
- Almuqhim, F.; Saeed, F. ASD-SAENet: A sparse autoencoder, and deep-neural network model for detecting autism spectrum disorder (ASD) using fMRI data. Front. Comput. Neurosci. 2021, 15, 654315. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, G.W.; Bong, G.; Yoo, H.J.; Kim, H.K. Deep-learning-based detection of infants with autism spectrum disorder using auto-encoder feature representation. Sensors 2020, 20, 6762. [Google Scholar] [CrossRef] [PubMed]
- Mujeeb Rahman, K.K.; Monica Subashini, M. A deep neural network-based model for screening autism spectrum disorder using the quantitative checklist for autism in toddlers (QCHAT). J. Autism Dev. Disord. 2022, 52, 2732–2746. [Google Scholar] [CrossRef] [PubMed]
- Saranya, A.; Anandan, R. FIGS-DEAF: An novel implementation of hybrid deep learning algorithm to predict autism spectrum disorders using facial fused gait features. Distrib. Parallel Databases 2022, 40, 753–778. [Google Scholar] [CrossRef]
- Shao, L.; Fu, C.; You, Y.; Fu, D. Classification of ASD based on fMRI data with deep learning. Cogn. Neurodyn. 2021, 15, 961–974. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Rashid, J.; Faheem, M.; Akram, A.; Khan, N.A.; Amin, R. Autism spectrum disorder detection using facial images: A performance comparison of pretrained convolutional neural networks. Healthc. Technol. Lett. 2024, 1–13. [Google Scholar] [CrossRef]
- Subah, F.Z.; Deb, K.; Dhar, P.K.; Koshiba, T. A deep learning approach to predict autism spectrum disorder using multisite resting-state fMRI. Appl. Sci. 2021, 11, 3636. [Google Scholar] [CrossRef]
- Tang, M.; Kumar, P.; Chen, H.; Shrivastava, A. Deep multimodal learning for the diagnosis of autism spectrum disorder. J. Imaging 2020, 6, 47. [Google Scholar] [CrossRef]
- Han, J.; Jiang, G.; Ouyang, G.; Li, X. A multimodal approach for identifying autism spectrum disorders in children. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 2003–2011. [Google Scholar] [CrossRef]
- Kong, Y.; Gao, J.; Xu, Y.; Pan, Y.; Wang, J.; Liu, J. Classification of autism spectrum disorder by combining brain connectivity and deep neural network classifier. Neurocomputing 2019, 324, 63–68. [Google Scholar] [CrossRef]
- Liu, J.; Sheng, Y.; Lan, W.; Guo, R.; Wang, Y.; Wang, J. Improved ASD classification using dynamic functional connectivity and multi-task feature selection. Pattern Recognit. Lett. 2020, 138, 82–87. [Google Scholar] [CrossRef]
- Arya, D.; Olij, R.; Gupta, D.K.; El Gazzar, A.; Wingen, G.; Worring, M.; Thomas, R.M. Fusing structural and functional MRIs using graph convolutional networks for autism classification. In Proceedings of the Third Conference on Medical Imaging with Deep Learning, PMLR, Montreal, QC, Canada, 6–8 July 2020; pp. 44–61. [Google Scholar]
- Eslami, T.; Mirjalili, V.; Fong, A.; Laird, A.R.; Saeed, F. ASD-DiagNet: A hybrid learning approach for detection of autism spectrum disorder using fMRI data. Front. Neuroinform. 2019, 13, 70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, C.; Li, P.; Peng, Y.; Kang, X.; Jiang, C.; Li, F.; Zhu, X.; Yao, D.; Biswal, B.; et al. Separated channel attention convolutional neural network (SC-CNN-attention) to identify ADHD in multi-site rs-fMRI dataset. Entropy 2020, 22, 893. [Google Scholar] [CrossRef]
- Mujeeb Rahman, K.K.; Subashini, M.M. Identification of autism in children using static facial features and deep neural networks. Brain Sci. 2022, 12, 94. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, D.; Huang, J.; Yap, P.T.; Shen, D.; Liu, M. Identifying autism spectrum disorder with multi-site fMRI via low-rank domain adaptation. IEEE Trans. Med. Imaging 2019, 39, 644–655. [Google Scholar] [CrossRef]
- Baygin, M.; Dogan, S.; Tuncer, T.; Barua, P.D.; Faust, O.; Arunkumar, N.; Abdulhay, E.W.; Palmer, E.E.; Acharya, U.R. Automated ASD detection using hybrid deep lightweight features extracted from EEG signals. Comput. Biol. Med. 2021, 134, 104548. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, D.; Tang, Y.; Zhang, L. Children ASD evaluation through joint analysis of EEG and eye-tracking recordings with graph convolution network. Front. Hum. Neurosci. 2021, 15, 651349. [Google Scholar] [CrossRef]
- Wang, C.; Xiao, Z.; Wang, B.; Wu, J. Identification of autism based on SVM-RFE and stacked sparse auto-encoder. IEEE Access 2019, 7, 118030–118036. [Google Scholar] [CrossRef]
- Abbas, S.Q.; Chi, L.; Chen YP, P. DeepMNF: Deep Multimodal Neuroimaging Framework for Diagnosing Autism Spectrum Disorder. Artif. Intell. Med. 2023, 136, 102475. [Google Scholar] [CrossRef]
- Rakhimberdina, Z.; Liu, X.; Murata, T. Population graph-based multi-model ensemble method for diagnosing autism spectrum disorder. Sensors 2020, 20, 6001. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, S.; Wu, F.X. Diagnosis of autism spectrum disorder with convolutional autoencoder and structural MRI images. In Neural Engineering Techniques for Autism Spectrum Disorder; Academic Press: Cambridge, MA, USA, 2021; pp. 23–38. [Google Scholar]
- Sherkatghanad, Z.; Akhondzadeh, M.; Salari, S.; Zomorodi-Moghadam, M.; Abdar, M.; Acharya, U.R.; Khosrowabadi, R.; Salari, V. Automated detection of autism spectrum disorder using a convolutional neural network. Front. Neurosci. 2020, 13, 1325. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).