Abstract
In this study, sawdust (SWS) was employed as an eco-friendly and low-cost adsorbent for the removal of anionic (Congo red, CR) and cationic (methylene blue, MB) dyes from aqueous solutions at 25 °C. The investigation encompasses various parameters affecting the adsorption process, including weight of sawdust adsorbent, pH, initial dye concentration, and equilibrium time. Characterization techniques such as Transmission Electron Microscopy (TEM) and Scanning Electron Microscopy (SEM) were conducted for an in-depth understanding of the adsorption mechanism. Optimal conditions were found to be SWS weight of 0.1 g/L, dye concentration of 15 mg/L, and equilibrium time of 1 h. Under these conditions, removal percentages of 95.88% for MB and 67.78% for CR were achieved, with adsorption capacities of 14.35 mg/g and 10.22 mg/g, respectively. The results demonstrate that SWS, though considered waste, has significant potential as a low-cost adsorbent for dye removal from aqueous solutions. Removal efficiency increased with SWS weight, ranging from 75.54% to 98.50% for MB, and 50.86% to 80.012% for CR, while adsorption capacity (Qe) inversely correlated with surface weight, ranging from 45.55 to 9.12 mg/g for MB, and 15.23 to 8.076 mg/g for CR.
1. Introduction
Adsorption is one of the pivotal techniques created by researchers for the removal of harmful chemicals from the environment. This method is widely used due to its effectiveness in selectively eliminating pollutants such as industrial dyes, medications, and heavy metals. Among these, dyes are particularly concerning as they are detrimental to both animal and human health, and their release into aquatic solutions can have severe environmental effects [1,2,3,4,5,6]. A common substance used in adsorption technology is activated carbon, prepared from readily available and inexpensive agricultural waste. This includes the peels of coconuts, pomegranates, watermelons, oranges, and palm fronds. Sawdust, a byproduct of various wood processing industries, constitutes over 40% of the lignocellulosic residues produced globally, totaling more than 180 million m3. However, sawdust is often undervalued and underutilized, leading to the need to transform this low-value waste into valuable products through sustainable industrial processing [6,7,8,9,10]. Congo red (CR) is a non-biodegradable dye that is harmful to both human health and aquatic life. Its persistent aromatic structure necessitates extensive treatment before release into the environment. Several physical, chemical, and biological treatments for CR have been studied, each with its unique challenges, advantages, disadvantages, applications, and costs [11,12,13].
Methylene blue (MB), widely used in various industries, also poses serious environmental and health threats when released into water bodies. Despite its therapeutic benefits in controlled doses, wastewater containing MB dye can lead to diseases in humans such as cyanosis, tissue necrosis, and an increased heart rate. Moreover, MB affects plant life, inhibiting growth and reducing pigment and protein content in certain microalgae species. Therefore, the thorough treatment of wastewater containing MB dye is essential before industrial discharge [14,15,16].
2. Materials and Methods
2.1. Chemicals
Methylene blue or Methylthioninium chloride (molecular mass: 319.85 g/mol, chemical formula: CHClNS) and Congo red (molecular mass: 696.665 g/mol, chemical formula: CHNNaOS) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Standard solutions of the dyes (500 mg/L) were prepared by dissolving 0.5 g in 500 mL of distilled water (DW).
2.2. Preparation of Adsorbent
Sawdust (SWS) was collected from a sawdust factory in Hill-Iraq. The untreated SWS was washed several times in distilled water and filtered to obtain clean untreated sawdust. The cleaned material was oven-dried for 24 h at 60 °C, ground, and sieved to a particle size range of to mm. The prepared SWS was stored in a glass bottle for further use, with no additional physical or chemical treatments applied prior to experiments.
2.3. Equilibrium Studies
Adsorption experiments were performed by adding varying weights of SWS ( to g) into 100 mL conical flasks containing different initial dye concentrations (5 to 25 mg/L) and pH solutions (3 to 10) at 25 °C. The flasks were placed in a water-bath shaker at 130 rpm for 60 min until equilibrium was reached. The concentrations of MB and CR dyes were measured using a double beam UV–vis spectrophotometer at 630 nm and 590 nm wavelengths, respectively. The adsorption capacity, (mg/g) and removal percentage % were calculated using Equations (1) and (2)
where and are the initial and equilibrium concentrations of the dye, V is the volume in milliliters, and W is the weight in grams.
3. Results and Discussion
3.1. Characterization of Adsorbent
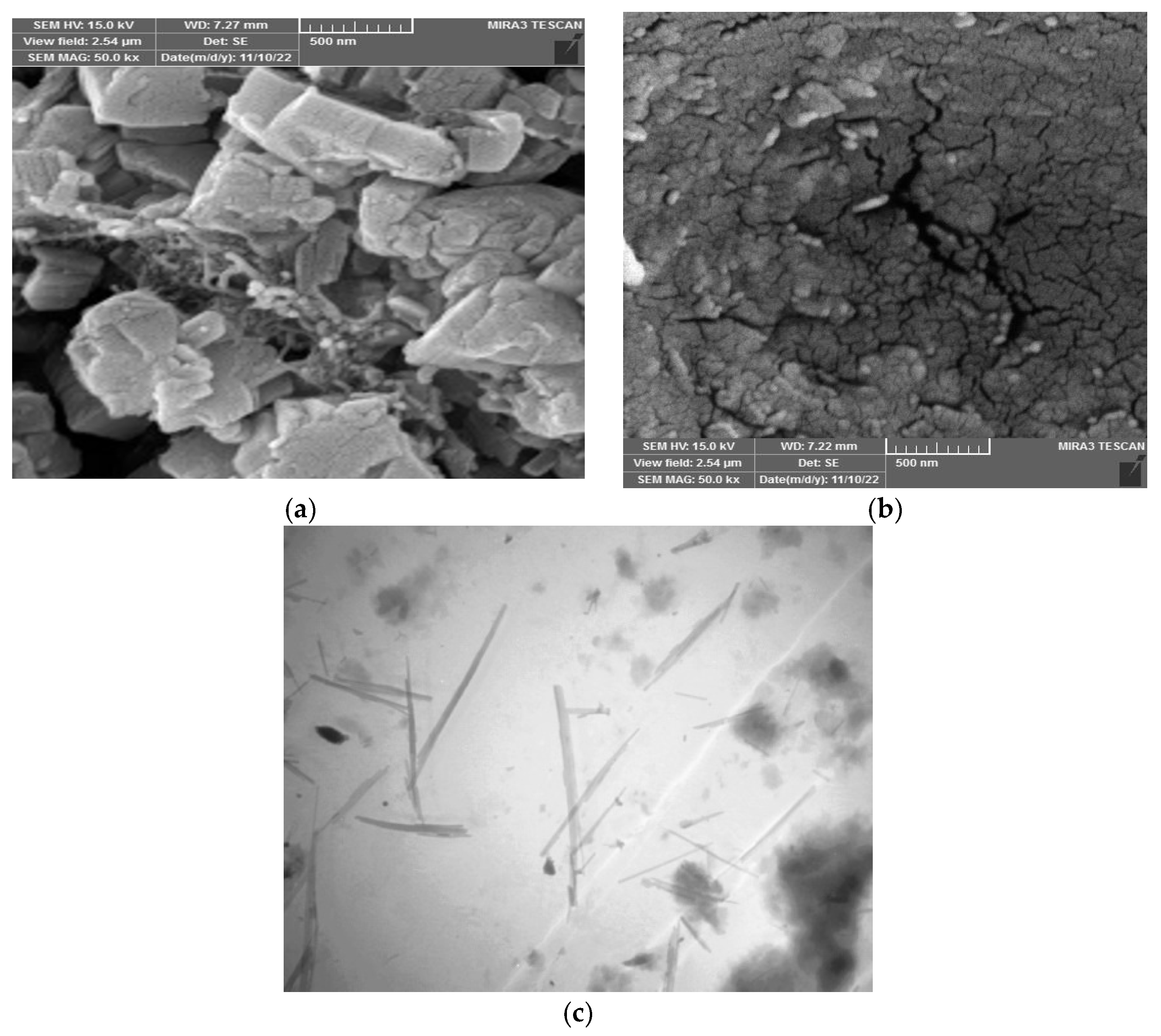
Figure 1 displays the Field Emission Scanning Electron Microscope (FESEM) and Transmission Electron Microscopy (TEM) images characterizing the sawdust (SWS) adsorbent. The FESEM image in Figure 1a reveals lamellar aggregates forming a porous and rough surface, enhancing the material’s absorbance capability by facilitating water diffusion. After adsorption, Figure 1b illustrates a change in surface morphology, such as the disappearance of surface roughness, indicating the fullness of active sites [16,17]. Figure 1c showcases dark spots via TEM, evidencing active sites contributing to adsorption and increased surface efficiency [18].
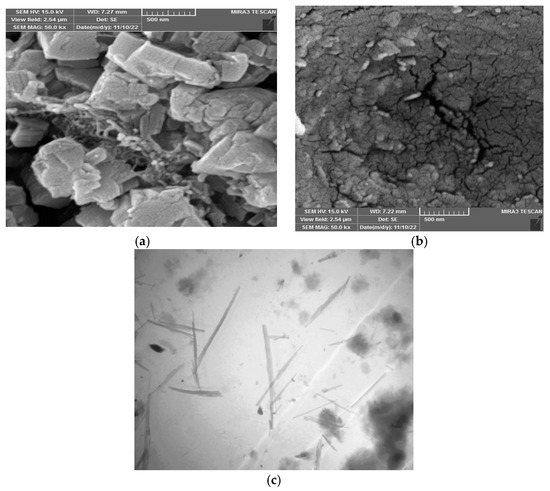
Figure 1.
FESEM: (a) SWS before adsorption, (b) SWS after adsorption, and TEM (c) SWS adsorbent.
3.2. Effect of Equilibrium Time
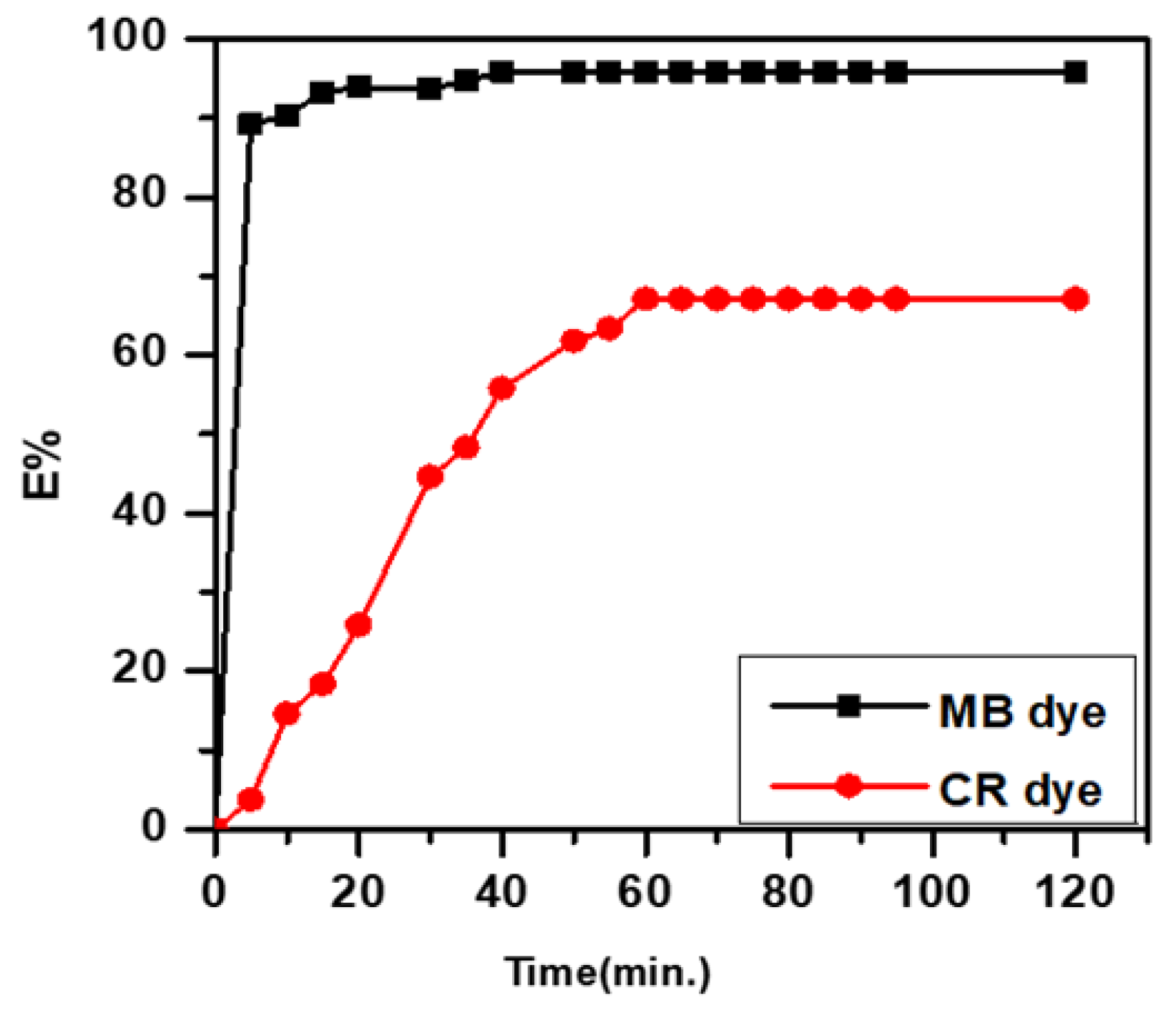
Figure 2 demonstrates the effect of equilibrium time on the removal of MB and CR dyes by SWS. The removal percentage increased over time, reaching equilibrium within 60 min for concentrations studied (15 mg/L). The adsorption curve’s steep initial increase indicates a high rate of adsorption, with optimum removal percentages of 97.77% for MB and 68.87% for CR dyes at 60 min. Hence, the best equilibrium time was selected as 60 min for all experiments [19,20,21].
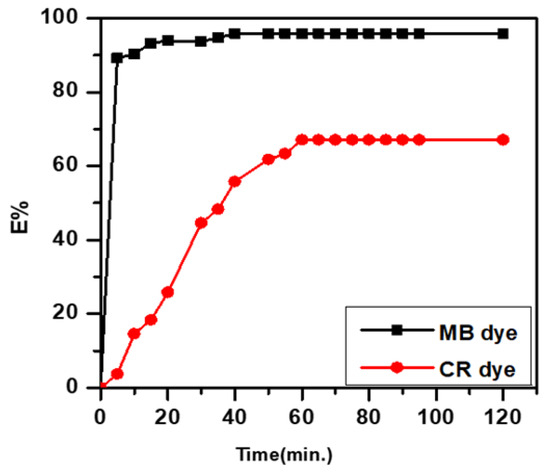
Figure 2.
Effect of equilibrium time.
3.3. Effect of Weight of SWS
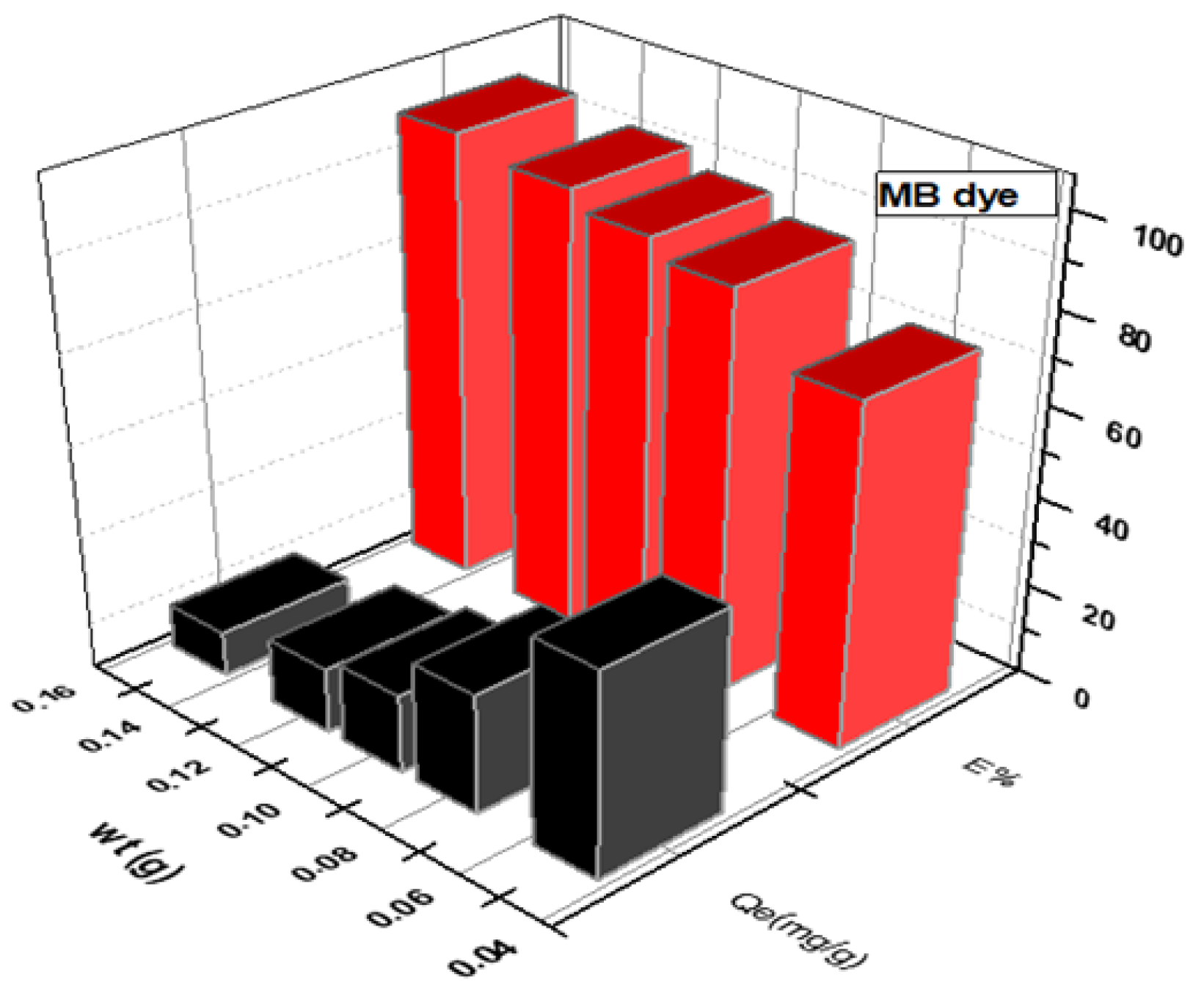
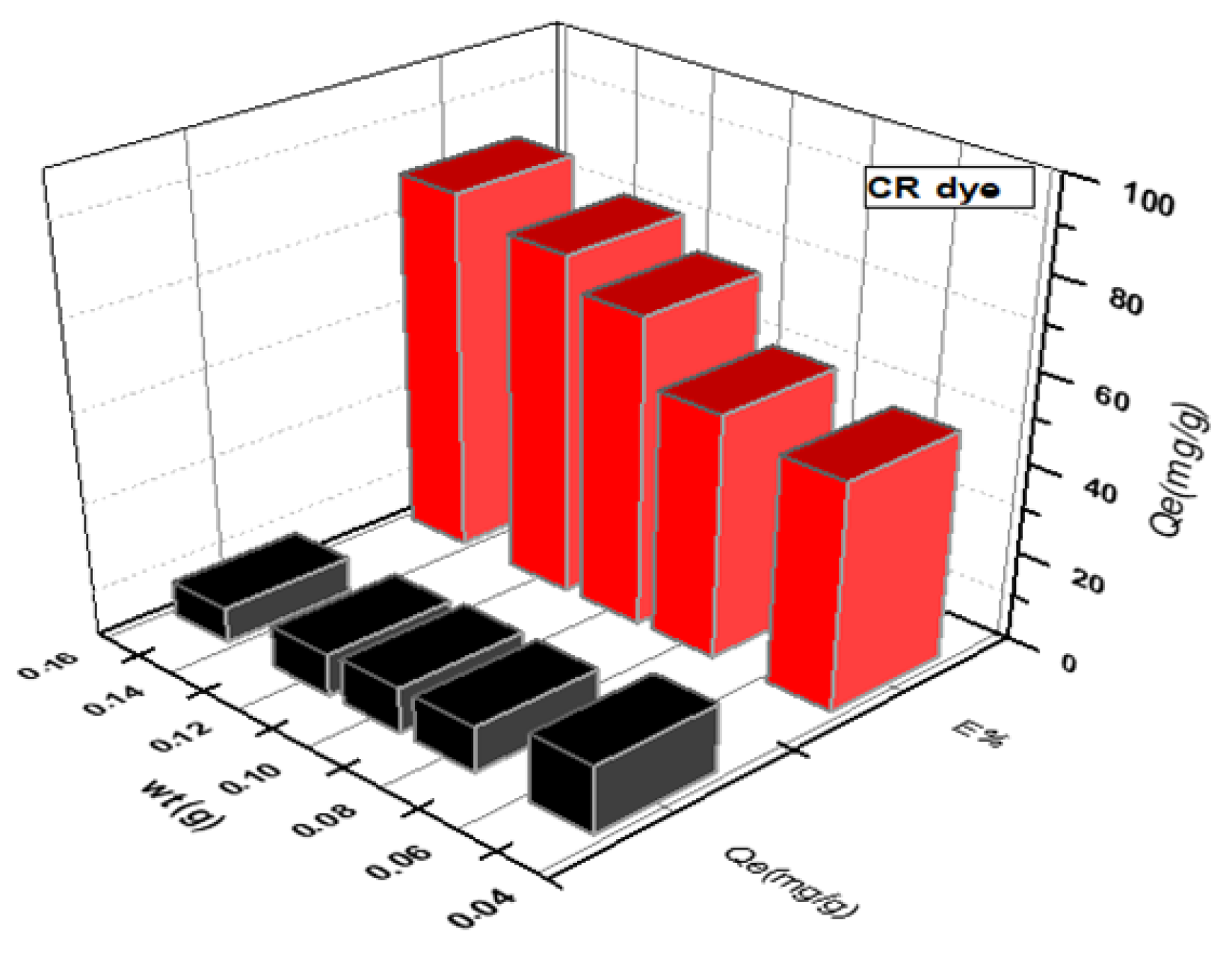
Figure 3 and Figure 4 depict the impact of SWS weight on MB and CR dye adsorption. An increase in SWS weight from to g corresponded to removal percentage increases from to for MB dye and to for CR dye. Conversely, adsorption efficiency (Qe) decreased with increasing surface weight, as shown in the results [16,22,23,24,25,26,27].
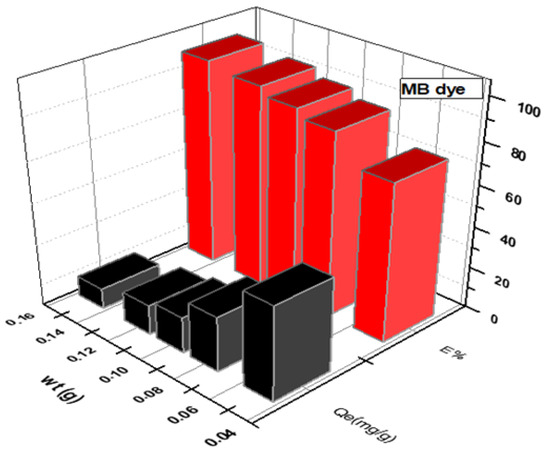
Figure 3.
Effect of weight of SWS surface on removal of cationic MB dye.
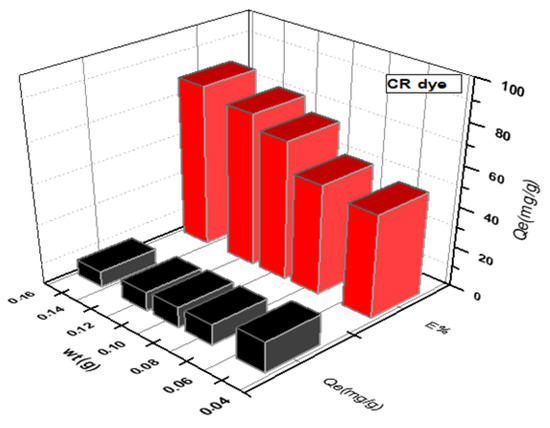
Figure 4.
Effect of weight of SWS surface on removal of anionic CR dye.
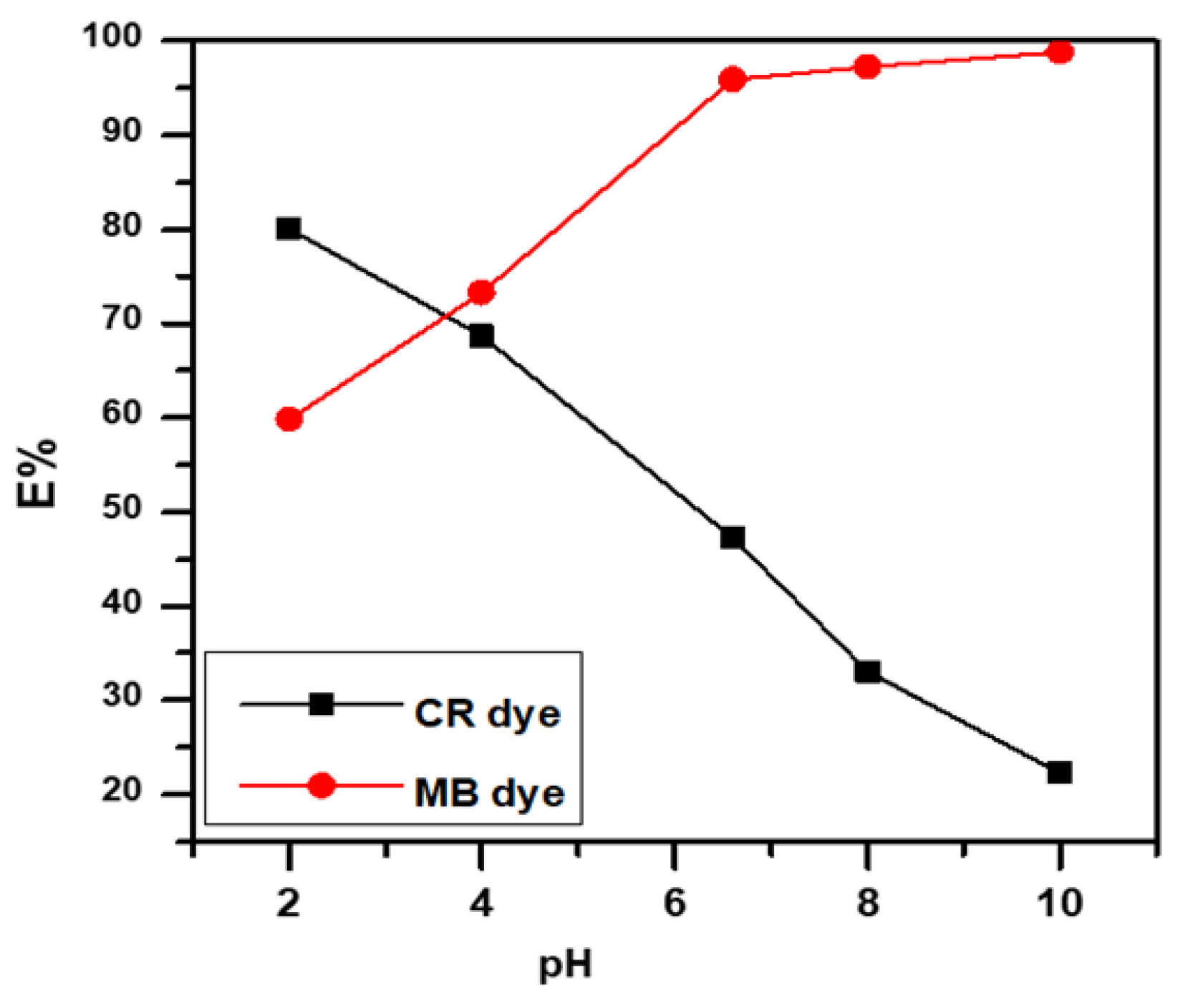
3.4. Effect of pH Solution
Figure 5 displays the extraordinary influence of pH on the adsorbent surface and MB and CR dye ionization. MB dye sorption was almost constant at pH 6 to 10, but decreased at pH 3, whereas CR dye showed better removal in acidic media, depending on the surface charge [17,18,20,28,29].
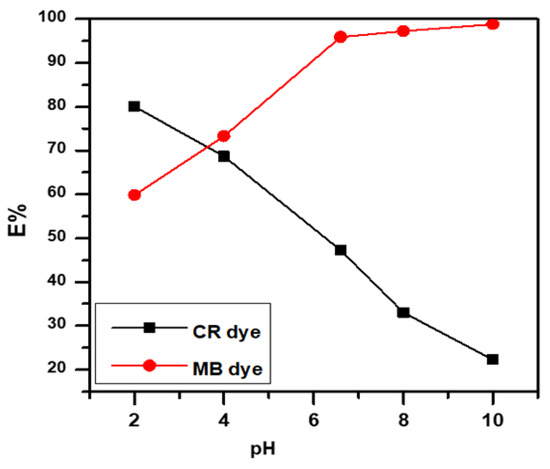
Figure 5.
Effect of pH solution on anionic CR dye and cationic MB dye.
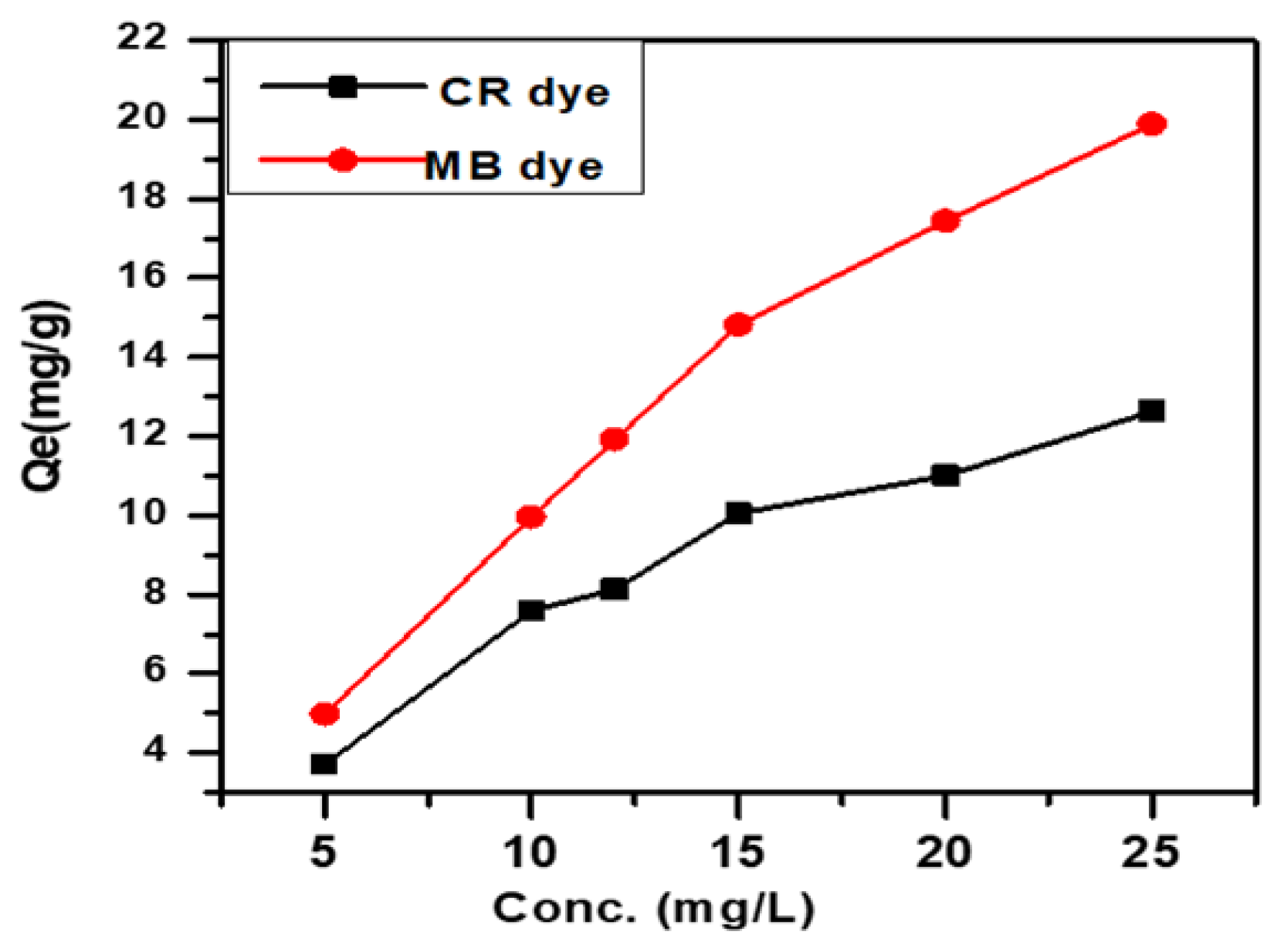
3.5. Effect of Initial Concentration of Anionic and Cationic Dyes
Figure 6 illustrates the influence of initial dye concentration on adsorption capacity (Qe mg/g) for dye concentrations between 5 and 25 mg L. The results show the adsorption efficiency for SWS at 15 mg/L concentration of CR and MB dyes, revealing the impact of initial concentration on adsorption performance [30].
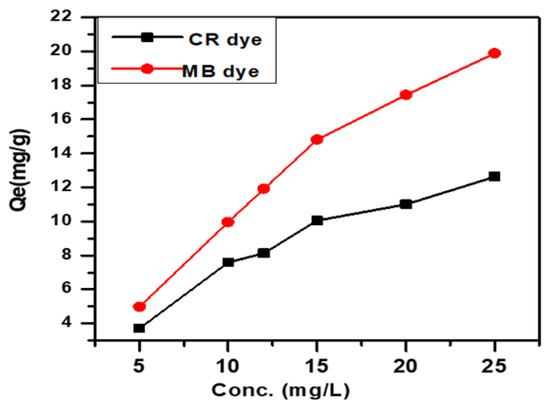
Figure 6.
Effect of initial concentration of anionic CR dye and cationic MB dye.
3.6. Conclusions
In this study, an environmentally friendly and cost-effective approach of utilizing sawdust as an adsorbent surface was explored without relying on any chemical treatment. This process was characterized by a detailed examination of various factors influencing the adsorption of MB and CR dyes. The optimal removal percentages achieved were 97.77% for MB dye and 68.87% for CR dye within a 60 min equilibrium time. The pH had distinct effects on the adsorption of the two dyes, with the sorption of MB dye being optimal at pH levels of 6–10, and CR dye performing best at pH 3. Additionally, the removal percentage increased with the weight of SWS, with the range of 0.05–0.15 g yielding a significant improvement in removal efficiency from 75.54% to 98.50% for MB dye and from 50.86% to 80.012% for CR dye. These findings underscore the potential of sawdust (SWS) as an accessible and promising adsorbent for the removal of dyes from water. Its effectiveness in adsorbing different dyes at various conditions emphasizes its versatility, presenting it as an attractive option for broader applications in water treatment. Further optimization and exploration of this method could contribute to sustainable and economically viable solutions for water purification and dye removal.
Author Contributions
Conceptualization, A.M.A. and A.F.A.; methodology, F.H.A.; validation, Z.M.S.; investigation, H.A.A.; resources, A.F.A.; writing—original draft preparation, A.M.A.; writing—review and editing, F.H.A.; visualization, Z.M.S.; supervision, H.A.A.; project administration, A.M.A. and A.F.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data used in this experiment have been made available in the present article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mariah, G.K.; Pak, K.S. Removal of brilliant green dye from aqueous solution by electrocoagulation using response surface methodology. Mater. Today Proc. 2020, 20, 488–492. [Google Scholar] [CrossRef]
- Khammas, Z.A.; Abdulkareem, H.M. A New Visible Spectrophotometric Approach for Mutual Determination of Amoxicillin and Metoclopramide Hydrochloride in Pharmaceuticals after Cloud Point Extraction. Sci. J. Anal. Chem. 2016, 4, 66–76. [Google Scholar] [CrossRef]
- Khedaer, Z.; Ahmed, D.; Al-Jawad, S. Investigation of Morphological, Optical, and Antibacterial Properties of Hybrid ZnO-MWCNT Prepared by Sol-gel. J. Appl. Sci. Nanotechnol. 2021, 1, 66–77. [Google Scholar] [CrossRef]
- Bunge, A.; Leoștean, C.; Turcu, R. Synthesis of a Magnetic Nanostructured Composite Sorbent Only from Waste Materials. Materials 2023, 16, 7696. [Google Scholar] [CrossRef]
- Ortega-Toro, R.; Villabona-Ortíz, Á.; Tejada-Tovar, C.; Herrera-Barros, A.; Cabrales-Sanjuan, D. Use of Sawdust (Aspidosperma polyneuron) in the Preparation of a Biocarbon-Type Adsorbent Material for Its Potential Use in the Elimination of Cationic Contaminants in Wastewater. Water 2023, 15, 3868. [Google Scholar] [CrossRef]
- Alqaragully, M.B.; AL-Gubury, H.Y.; Aljeboree, A.M.; Karam, F.F.; Alkaim, A.F. Monoethanolamine: Production plant. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 1287–1296. [Google Scholar]
- Liu, W.; Zhang, S.; Liu, K.; Yang, H.; Lin, Q.; Xu, T.; Song, X.; Du, H.; Bai, L.; Yao, S.; et al. Sustainable preparation of lignocellulosic nanofibrils and cellulose nanopaper from poplar sawdust. Clean. Prod. 2023, 384, 135582. [Google Scholar] [CrossRef]
- Shi, X.; Wang, W.; Kang, Y.; Wang, A. Enhanced Swelling Properties of a Novel Sodium Alginate-Based Superabsorbent Composites: NaAlg-g-poly(NaA-co-St)/APT. J. Appl. Polym. Sci. 2012, 125, 1822–1832. [Google Scholar] [CrossRef]
- Velić, N.; Stjepanović, M.; Pavlović, S.; Bagherifam, S.; Banković, P.; Jović-Jovičić, N. Modified Lignocellulosic Waste for the Amelioration of Water Quality: Adsorptive Removal of Congo Red and Nitrate Using Modified Poplar Sawdust. Water 2023, 15, 3776. [Google Scholar] [CrossRef]
- Mansour, R.A.; El Shahawy, A.; Attia, A.; Beheary, M.S. Brilliant Green Dye Biosorption Using Activated Carbon Derived from Guava Tree Wood. Int. J. Chem. Eng. 2020, 2, 190. [Google Scholar] [CrossRef]
- Oladoye, P.O.; Bamigboye, M.O.; Ogunbiyi, O.D.; Akano, M.T. Toxicity and decontamination strategies of Congo red dye. Groundw. Sustain. Dev. 2022, 19, 100844. [Google Scholar] [CrossRef]
- Shalah, L.A.M.; Ali, S.S.; Al, Z.S.; Hindi, F.A.; Alkaim, A.F. Screen the efficient growth of e.Coli to removal congo red dye by some modified media. Int. J. Pharm. Res. 2019, 11, 496–500. [Google Scholar]
- Omran, A.R.; Baiee, M.A.; Juda, S.A.; Salman, J.M.; AlKaim, A.F. Removal of congo red dye from aqueous solution using a new adsorbent surface developed from aquatic plant (Phragmites australis). Int. J. Chemtech Res. 2016, 9, 334–342. [Google Scholar]
- Oladoye, P.O.; Ajiboye, T.O.; Omotola, E.O.; Oyewola, O.J. Methylene blue dye: Toxicity and potential elimination technology from wastewater. Results Eng. 2022, 16, 100678. [Google Scholar] [CrossRef]
- Sivaranjani, K.; Kaliyappan, S.; Sivakumar, S.; Dharmaraja, J. Enhancement Photocatalytic Activity of Mn Doped CdS/ZnO Nanocomposites for the Degradation of Methylene Blue Under Solar Light Irradiation. Adv. Mater. Sci. 2022, 22, 28–48. [Google Scholar] [CrossRef]
- Thakur, S.; Chaudhary, J.; Thakur, A.; Gunduz, O.; Alsanie, W.F.; Makatsoris, C.; Thakur, V.K. Highly efficient poly(acrylic acid-co-aniline) grafted itaconic acid hydrogel: Application in water retention and adsorption of rhodamine B dye for a sustainable environment. Chemosphere 2022, 303, 134917. [Google Scholar] [CrossRef] [PubMed]
- Aljeboree, A.; Essa, S.; Kadam, Z.; Dawood, F.; Falah, D.; AF, A. Environmentally Friendly Activated Carbon Derived from Palm Leaf for the Removal of Toxic Reactive Green Dye. Int. J. Pharm. Qual. Assur. 2023, 14, 12–15. [Google Scholar] [CrossRef]
- Pashaei-Fakhri, S.; Peighambardoust, S.J.; Foroutan, R.; Arsalani, N.; Ramavandi, B. Crystal violet dye sorption over acrylamide/graphene oxide bonded sodium alginate nanocomposite hydrogel. Chemosphere 2021, 270, 129419. [Google Scholar] [CrossRef]
- Alkaim, A.F.; Ajobree, A.M. White marble as an alternative surface for removal of toxic dyes (Methylene blue) from Aqueous solutions. Int. J. Adv. Sci. Technol. 2020, 29, 5470–5479. [Google Scholar]
- El Hajam, M.; Idrissi Kandri, N.; Özdemir, S.; Plavan, G.; Ben Hamadi, N.; Boufahja, F.; Zerouale, A. Statistical Design and Optimization of Cr (VI) Adsorption onto Native and HNO3/NaOH Activated Cedar Sawdust Using AAS and a Response Surface Methodology (RSM). Molecules 2023, 28, 7271. [Google Scholar] [CrossRef]
- Ilgin, P.; Ozay, H.; Ozay, O. Selective adsorption of cationic dyes from colored noxious effluent using a novel N-tert-butylmaleamic acid based hydrogels. React. Funct. Polym. 2019, 124, 189–198. [Google Scholar] [CrossRef]
- Aljeboree, A.M.; Alkaim, A.F. Comparative removal of three textile dyes from aqueous solutions by adsorption: As a model (corn-cob source waste) of plants role in environmental enhancement. Plant Arch. 2019, 19, 1613–1620. [Google Scholar]
- Xu, C.; Liu, R.; Tang, Q.; Hou, Y.; Chen, L.; Wang, Q. Adsorption Removal of Phosphate from Rural Domestic Sewage by Ca-Modified Biochar Derived from Waste Eggshell and Sawdust. Water 2023, 15, 3087. [Google Scholar] [CrossRef]
- Thakur, S. Synthesis, characterization and adsorption studies of an acrylic acid-grafted sodium alginate-based TiO2 hydrogel nanocomposite. Adsorpt. Sci. Technol. 2018, 36, 458–477. [Google Scholar] [CrossRef]
- Radia, N.D.; Kamona, S.M.H.; Jasem, H.; Abass, R.R.; Izzat, S.E.; Ali, M.S.; Ghafel, S.T.; Aljeboree, A.M. Role of Hydrogel and Study of its High-Efficiency to Removal Streptomycin drug from Aqueous Solutions. Int. J. Pharm. Qual. Assur. 2022, 13, 160–163. [Google Scholar]
- Gao, B.; Yu, H.; Wen, J.; Zeng, H.; Liang, T.; Zuo, F.; Cheng, C. Super-adsorbent poly(acrylic acid)/laponite hydrogel with ultrahigh mechanical property for adsorption of methylene blue. J. Environ. Chem. Eng. 2021, 9, 106346. [Google Scholar] [CrossRef]
- Pakdel, P.M.; Peighambardoust, S.J.; Foroutan, R.; Arsalani, N.; Aghdasinia, H. Decontamination of Fuchsin dye by carboxymethyl cellulose-graft-poly(acrylic acid-co-itaconic acid)/carbon black nanocomposite hydrogel. Int. J. Biol. Macromol. 2022, 222, 2083–2097. [Google Scholar] [CrossRef] [PubMed]
- Aljeboree, A.M.; Radia, N.D.; Jasim, L.S.; Alwarthan, A.A.; Khadhim, M.M.; Salman, A.W.; Alkaim, A.F. Synthesis of a new nanocomposite with the core TiO2/hydrogel: Brilliant green dye adsorption, isotherms, kinetics, and DFT studies. J. Ind. Eng. Chem. 2022, 109, 475–485. [Google Scholar] [CrossRef]
- Saif, M. Adsorption of Brilliant Green dye from aqueous solution onto red clay. Chem. Eng. J. 2020, 228, 54–62. [Google Scholar]
- Bader, A.T.; Aljeboree, A.M.; Alkaim, A.F. Removal of Methyl Violet (MV) from aqueous solutions by adsorption using activated carbon from pine husks (plant waste sources). Plant Arch. 2019, 19, 898–901. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).