Abstract
Titanium dioxide nanoparticles were prepared via the hydrothermal method, and silver was supported on Ti nanoparticles to form Ag/Ti using the photoreduction method. The prepared samples were dried overnight at and then calcined at for 2 h. Structural and morphological characterization were carried out using X-ray diffraction, field emission scanning electron microscopy (FE-SEM), and transmission electron microscopy (TEM). The adsorption performance and photocatalytic activity of the Ag/Ti were investigated using malachite green dye (MG) as a model organic pollutant in water. Along the way, the effects of various parameters were examined, such as regeneration experiments and removal of a laboratory sample (a mixture of several dyes) from aqueous solutions. The photocatalytic degradation efficiency reached 83.9%, 78.8%, and 68.5% during three cycles, compared to the standard solution (fresh), which reached 90.9%. These results underscore the potential application of Ag/Ti in environmental remediation, particularly in the degradation of organic dyes.
1. Introduction
The textile dyes, along with a large number of industrial pollutants, are highly toxic and potentially carcinogenic. Consequently, they are associated with environmental degradation and various diseases in animals and humans [1,2,3,4,5]. There has been a growing focus on dye degradation in industrial wastewaters, and several remediation strategies have been proposed. Pollutants have been eliminated using conventional physical techniques such as adsorption on activated carbon, ultrafiltration, and reverse osmosis [6,7,8]. However, these methods only succeed in transferring organic compounds from water to another phase, thus creating secondary pollution. Chemical methods such as chlorination generate carcinogenic and mutagenic byproducts that threaten human health [6]. An almost ideal solution to this problem is photocatalytic degradation. Specifically, dyes can degrade when exposed to visible light in the presence of a photocatalyst, due to their absorption in this spectrum. Because metal oxides are widely used in organic synthesis and have numerous environmental applications, there has been substantial interest in heterogeneous photodegradation by metal oxide in recent years [9,10]. Among the metal oxide semiconductors, titanium dioxide (TiO2) is one of the most effective photocatalysts currently in use. Its strong oxidizing power, nontoxicity, and long-term physical and chemical stability contribute to its effectiveness [11,12,13,14]. TiO2 has a wide band gap of 3.2 eV and requires only UV light for excitation to form photoactive species of holes (h+) and electrons (e−). The (h+) acts as a strong oxidizing agent, while the (e−) works as a reducing agent [15,16]. The recombination of h+ and e− in the TiO2 photocatalyst is rapid, resulting in an inefficient photocatalytic process. To overcome this limitation, TiO2 is often loaded with noble metals such as Au, Pd, Pt, and Ag.
Among these, Ag is the most used in doping TiO2, enhancing electron–hole separation, interfacial charge transfer ability, and increasing the visible light excitation of TiO2 nanoparticles (NPs) [17,18]. In this work, titanium dioxide is prepared by the hydrothermal method and silver-modified TiO2 (Ag/TiO2) is prepared by photoreduction. The obtained photocatalysts are characterized by means of X-ray diffraction, field emission scanning electron microscopy (FE-SEM), transmission electron microscopy (TEM), and energy dispersive X-ray spectroscopy (EDX). Malachite green (MG) is used as a model pollutant to study the photocatalytic activity of Ag/TiO2 under UV light irradiation.
2. Materials and Methods
2.1. Materials
The chemicals used in this study include titanium(IV) bis(ammonium lactato) dihydroxide (TALH, 50% aqueous solution), aqueous ammonia solution (25% NH3), ethanol 99.9%, and Malachite green dye (MG). All chemicals were used directly without further purification.
2.2. Preparation of Titanium Dioxide by Hydrothermal Method
Titanium dioxide (TiO2) nanoparticles (NPs) were prepared via the hydrothermal method using TALH. A mixture of 10 mL of an aqueous titanium(IV) bis(ammonium lactato) dihydroxide solution, 15 mL of an aqueous ammonia solution, and 40 mL ethanol was prepared, followed by the addition of distilled water to reach a final volume of 115 mL. This solution was mixed well using magnetic stirring for 30 min, then transferred into a Teflon cup, sealed in an autoclave, and placed into an electric furnace held at 180 °C for 24 h. The autoclave was then naturally cooled in air, washed several times with distilled water, and dried overnight at 60 °C. The product powder was calcined at a temperature of 500 °C for 2 h in a furnace, resulting in TiO2 nanoparticles.
2.3. Preparation of Silver Ag-Doped TiO2 Nanoparticles
Silver (Ag)-doped TiO2 nanoparticles were prepared by suspending 1 g of TiO2 NPs powder in 100 mL of distilled water and 2.5 mL of AgNO3 (0.01 M). This mixture was sonicated for 5 min and exposed to nitrogen gas for 10 min. Subsequently, 1.5 mL of methanol was added, and the mixture was irradiated with a light intensity of 1.71 mW/cm2 (using a UVA LED lamp) overnight, with continuous stirring. The resulting powder was washed several times with distilled water and dried for 24 h at 60 °C to obtain the Ag/TiO2 nanocomposite.
2.4. Preparation of Standard Solution of Malachite Green (MG) Dye
A stock solution (1000 mg/L) of aqueous MG dye was prepared by dissolving 1 g of MG in distilled water and then making up the solution to 1000 mL with distilled water. The solution’s maximum wavelength was found at MG = 625 nm. Additionally, 200 mL laboratory samples of dye pollutants containing multi-mixtures of various dyes were prepared for application in real samples.
2.5. Photo Catalysis Experiment
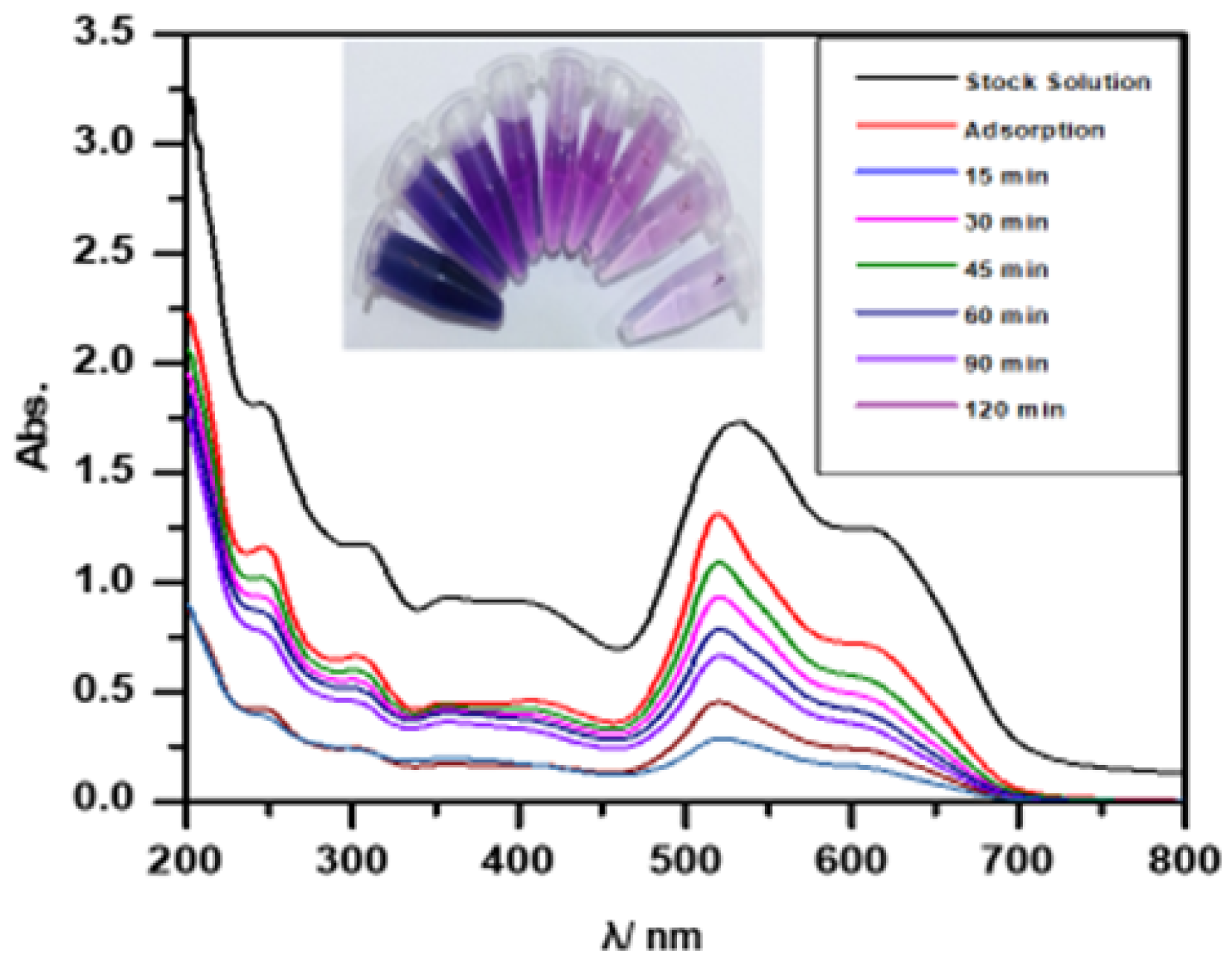
An established concentration of Malachite green (MG) solution (50 mg/L) was used to study the photocatalytic degradation. This solution was supplemented with 0.3 g of the nanocomposite and then transferred into the photoreaction vessel. It was continuously stirred while being exposed to UV light at 365 nm with an intensity of 1.27 MW/cm2. The solution was removed and centrifuged for 10 min at 3500 rpm after every interval of 10 min. The degradation of the centrifuged solution was measured using a UV–Vis spectrophotometer at its maximum absorbance wavelength of MG dye = 625 nm, and the result was studied.
3. Result and Discussion
3.1. XRD Diffraction of Ti and Ag/Ti Nanocomposite
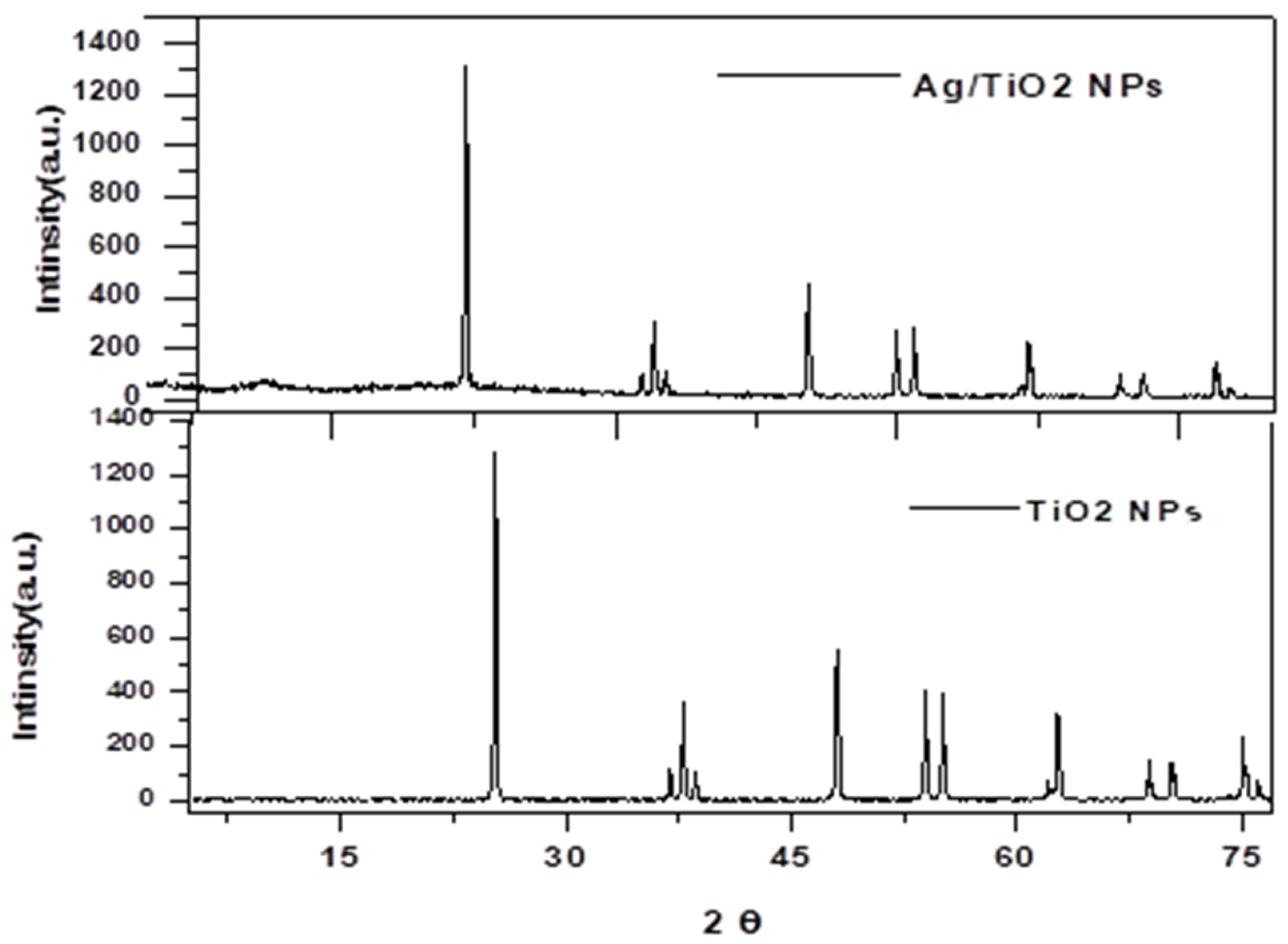
In Figure 1 the XRD patterns of prepared Ti show no other peaks that might indicate the presence of impurities, due to the effects of calcination at . The diffraction peaks were observed at , , , , , , and , which correspond to the (101), (108), (004), (112), (211), (200), (106) and (212) lattice planes of anatase Ti (JCPDS card no. 21-1272). The crystal shape of the diffraction peaks appears clear and sharp [19,20].
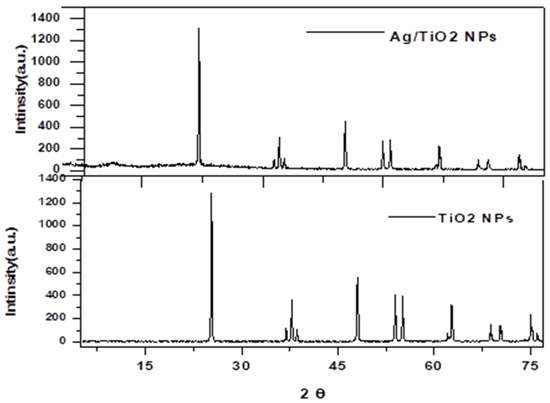
Figure 1.
XRD diffraction Ti and Ag/Ti nanocomposite.
Also we can notice in Figure 1, no crystalline phase involving Ag could be observed, indicating that the Ag is either too dispersed in the Ti lattice for clear crystal formation or that the Ag content is below the detection limit. Interestingly, no Ag crystal phase was detected; thus, at low Ag content, higher dispersion of smaller Ag nanoparticles on the surface and pores is clearly achieved [21].
3.2. FE-SEM Image of Ti and Ag/Ti Nanocomposite
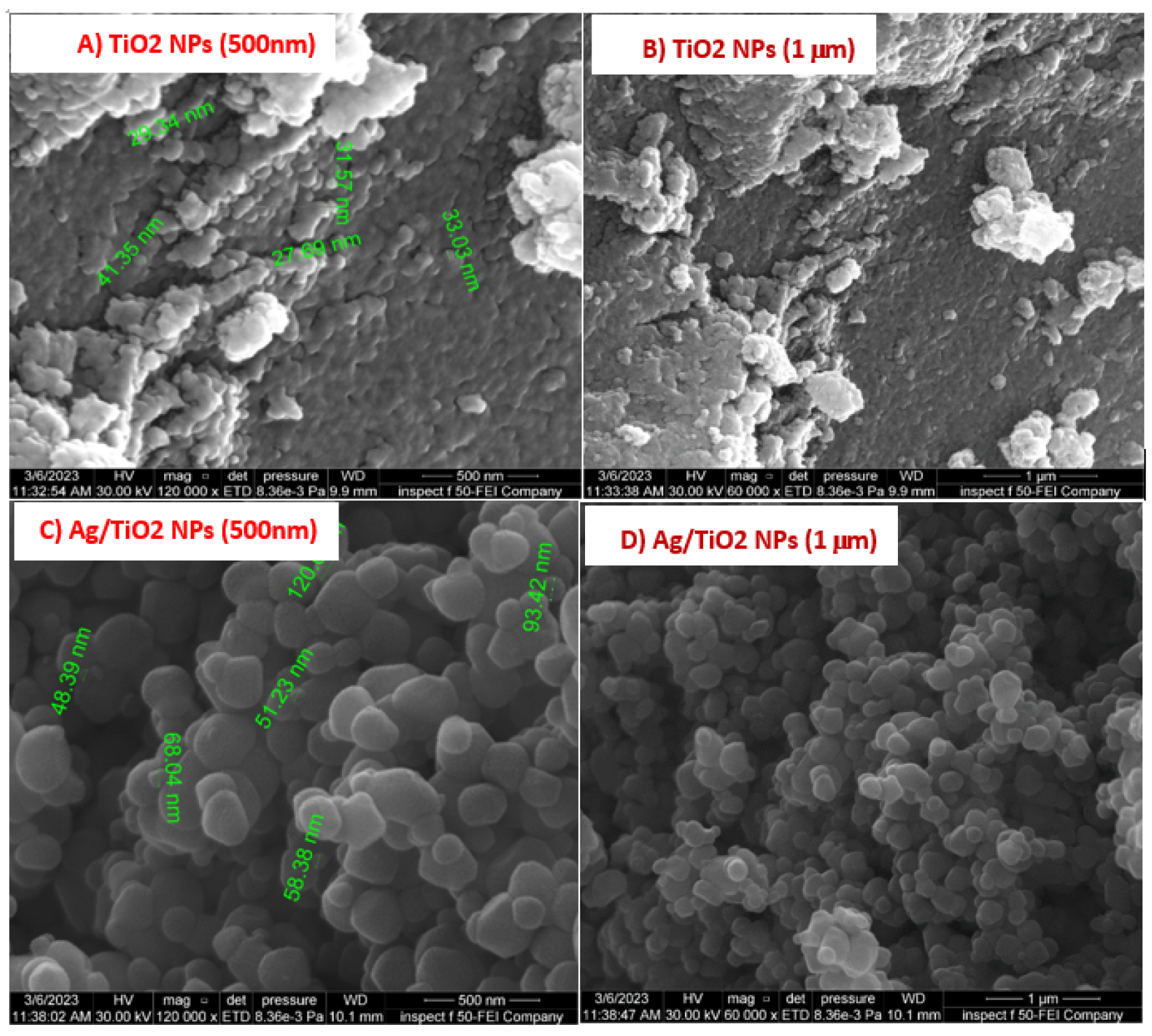
The surface morphology of pure Ti nanoparticles was studied using SEM, focusing on the size, shape of particles, and clusters among them, in addition to the distribution of these particles. Figure 2A,B show an SEM image of Ti, where the nanoparticles exhibit an anatase shape with a mean size of between 29.34–41.34 nm, with a high degree of agglomeration. Ag/TiO2 Figure 2C,D were found to overlap with Ti NPs, exhibiting cubic-like morphology with particle sizes ranging from around 48.34–120 nm [22,23].
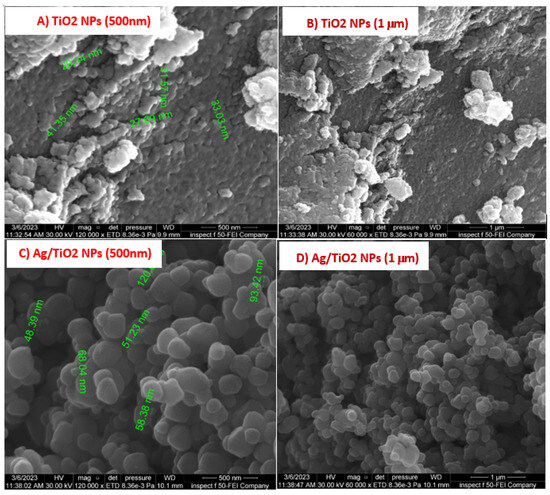
Figure 2.
FE-SEM images (A,B) Ti NPs in different magnitude, (C,D) Ag/Ti nanocomposite in different magnitude.
3.3. TEM and EDX of Ti and Ag/Ti Nanocomposite
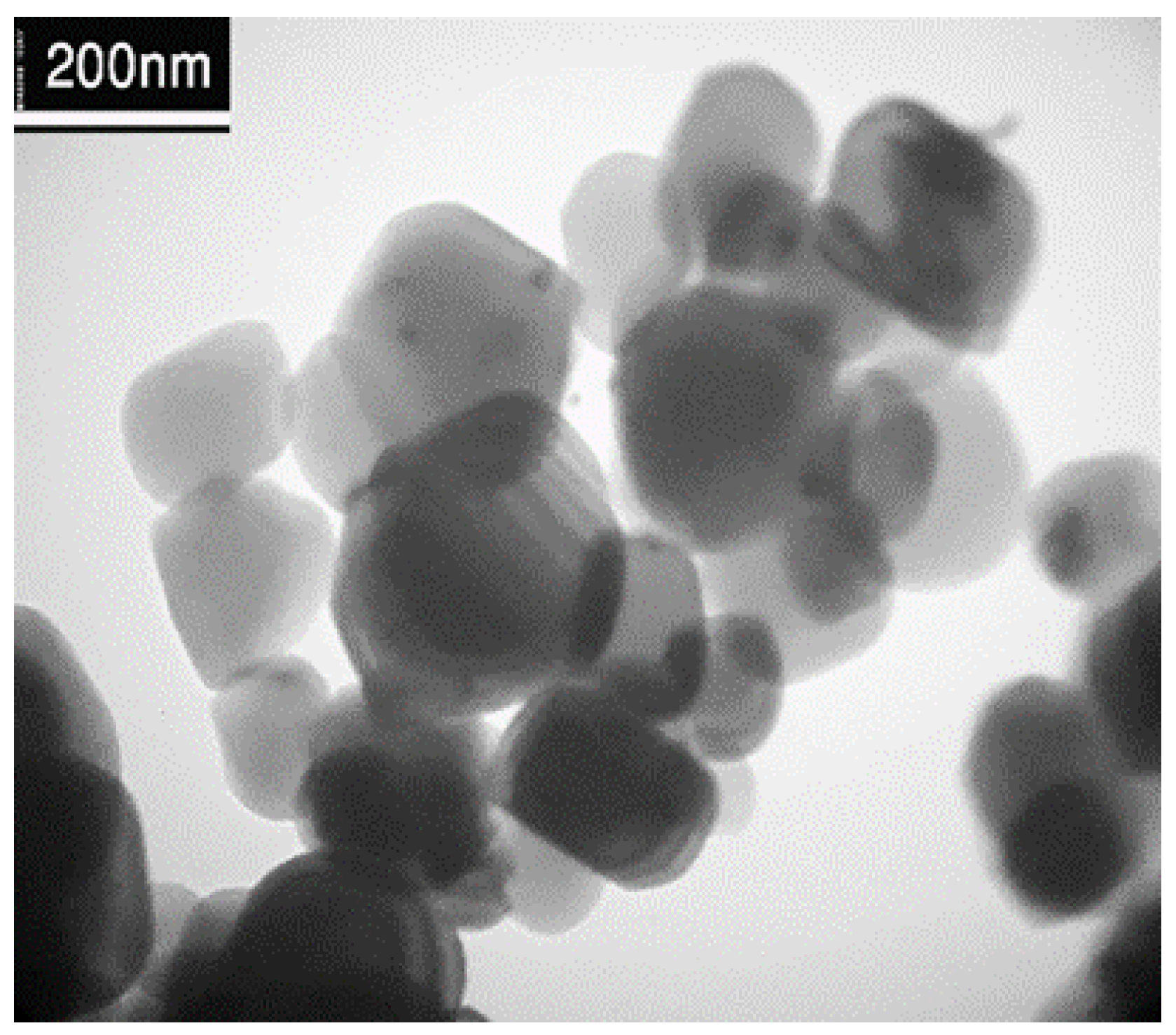
Transmission electron microscopy provides a more accurate view of the structure of prepared samples, such as crystallinity, distribution, and particle size. Figure 3 reveals that silver nanoparticles were clearly observed as the most intense dark spots on the Ti surface. The Ag nanoparticles deposited on the Ti are small and well deposited on the Ti NPs [24].
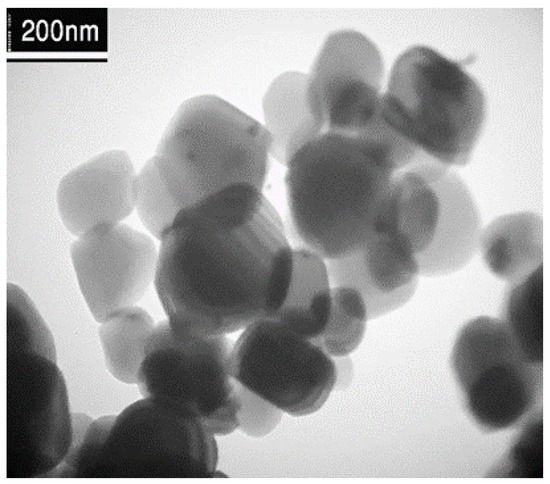
Figure 3.
TEM of image Ag/Ti.
3.4. Application of Prepared Nanocomposite
3.4.1. Recycling of the Photocatalyst Ag/Ti Nanocomposite
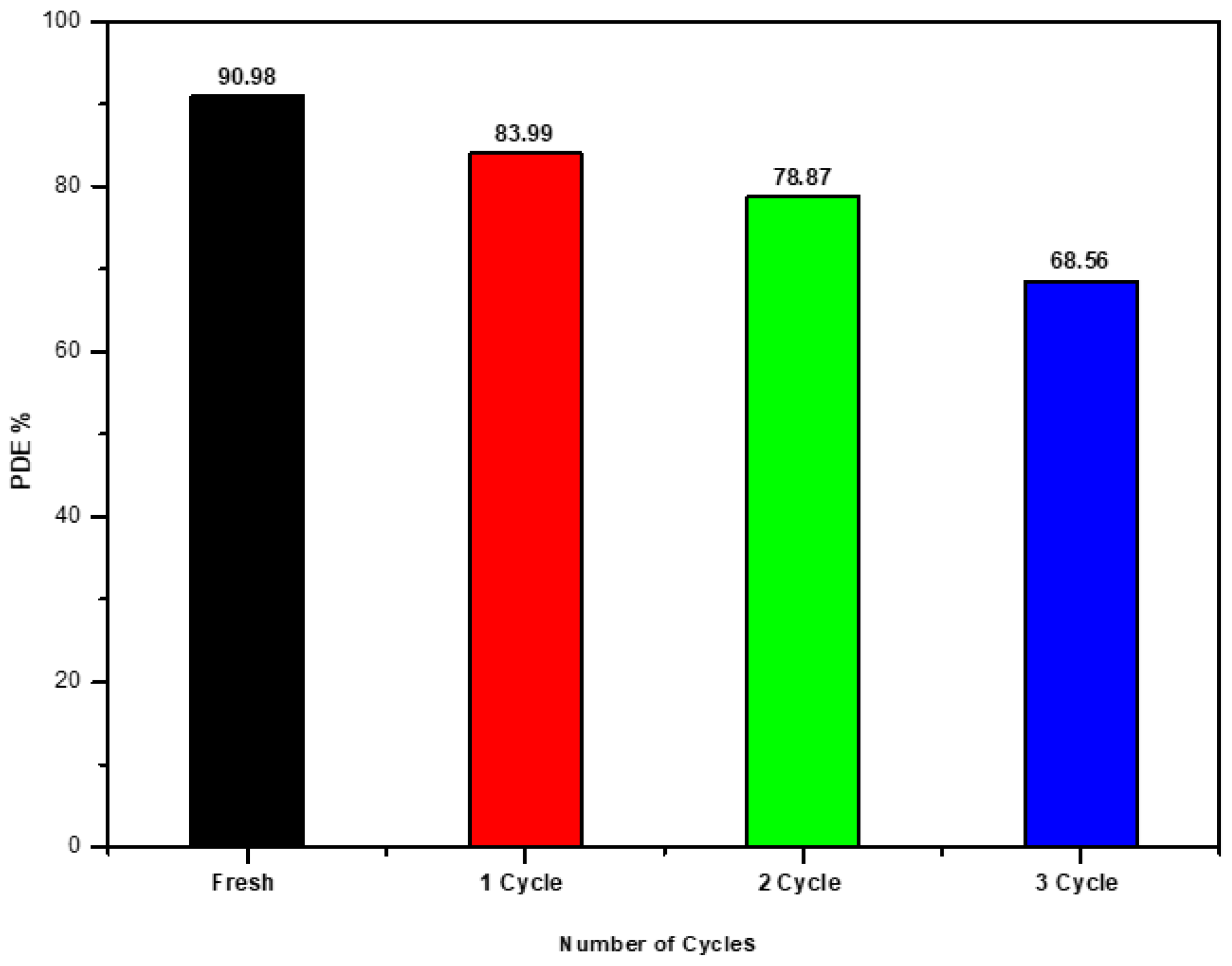
Recycling the catalyst is crucial in assessing the practical application of photocatalysts and developing heterogeneous photocatalysis technology for wastewater treatment. An examination of the photocatalytic activity of the recycled Ag/Ti nanocomposite catalyst on Malachite green dye shows degradation efficiencies of , , and during three cycles as shown Figure 4, compared to a standard solution (fresh) of . The photocatalysts exhibit self-cleaning surface properties and reusability, making them effective and promising for environmental remediation [25,26].
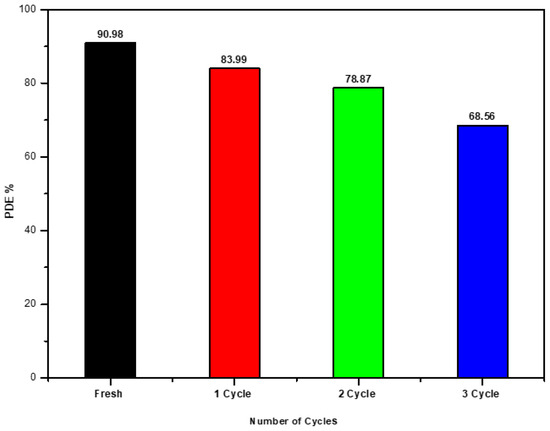
Figure 4.
Reusability of the photocatalyst for photocatalytic degradation MG dye.
3.4.2. Removal of Pollutants (Mixture of Dyes) by Using Ag/Ti Nanoparticles
A laboratory sample (200 mL) containing various dye pollutants was treated with 0.3 g of prepared Ag/Ti nanoparticles. The mixture was exposed to ultraviolet light for 2 h while maintaining a controlled distance from the light source, followed by centrifugation to separate the supernatant (shown in Figure 5). The remaining concentration was determined using UV–visible spectrophotometry. As time increased, absorption decreased, resulting in higher percentage removal [23].
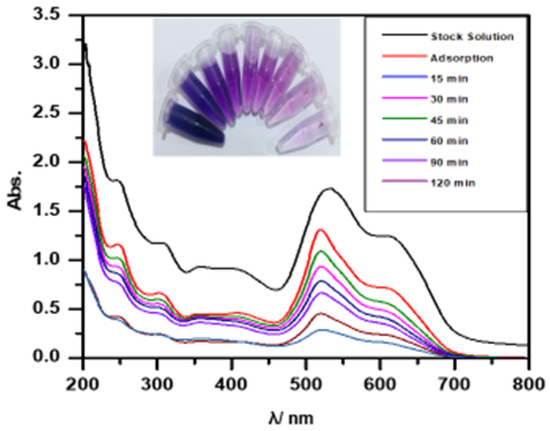
Figure 5.
Effect of removal of real sample (mixture of dyes) by using Ag/Ti NPs. Experimental conditions: mass amount 0.3 g/L, initial concentration 50 mg/L, temperature , and light intensity 2.1 mW·.
4. Conclusions
Titanium dioxide nanoparticles were prepared by the hydrothermal method, and further Ag/Ti was prepared by the photoreduction of silver on prepared Ti nanoparticles. These materials were characterized using XRD, FE-SEM, and TEM techniques. The XRD analysis concluded that the prepared Ti is a pure anatase phase. The study encompasses an investigation into various parameters such as regeneration experiments and removal of a laboratory sample, including a mixture of several dyes, from an aqueous solution. The results reveal that the photocatalysts have self-cleaning surface properties and reusability, making them effective. The photocatalyst is fundamentally stable and shows promise for environmental remediation.
Author Contributions
Conceptualization, A.M.A. and A.F.A.; methodology, Z.S.M.; validation, F.A.R.; investigation, N.A.A.S.; resources, A.F.A.; writing—original draft preparation, A.M.A.; writing—review and editing, F.A.R.; visualization, A.F.A.; supervision, N.A.A.S.; project administration, A.M.A. and A.F.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data used in the experiment have been made available in the present article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Saini, R.D. Textile organic dyes: Polluting effects and elimination methods from textile waste water. Int. J. Chem. Eng. Res. 2017, 9, 121–136. [Google Scholar]
- Karim, M.E.; Dhar, K.; Hossain, M.T. Decolorization of textile reactive dyes by bacterial monoculture and consortium screened from textile dyeing effluent. J. Genet. Eng. Biotechnol. 2018, 16, 375–380. [Google Scholar] [CrossRef]
- Aljeboree, A.M.; Alkaim, A.F. Role of plant wastes as an ecofriendly for pollutants (crystal violet dye) removal from aqueous solutions. Plant Arch. 2019, 19, 902–905. [Google Scholar]
- Alkaim, A.F.; Ajobree, A.M. White marble as an alternative surface for removal of toxic dyes (Methylene blue) from Aqueous solutions. Int. J. Adv. Sci. Technol. 2020, 29, 5470–5479. [Google Scholar]
- Rashad, S.; Zaki, A.; Farghali, A. Morphological effect of titanate nanostructures on the photocatalytic degradation of crystal violet. Nanomater. Nanotechnol. 2019, 9, 1847980418821778. [Google Scholar] [CrossRef]
- Das, S.; Mahalingam, H. Novel immobilized ternary photocatalytic polymer film based airlift reactor for efficient degradation of complex phthalocyanine dye wastewater. J. Hazard. Mater. 2020, 383, 121219. [Google Scholar] [CrossRef] [PubMed]
- Kaneva, N.; Bojinova, A.; Papazova, K. Enhanced Removal of Organic Dyes Using Co-Catalytic Ag-Modified ZnO and TiO2 Sol-Gel Photocatalysts. Catalysts 2023, 13, 245. [Google Scholar] [CrossRef]
- Saad Algarni, T.; Abduh, N.A.; Al Kahtani, A.; Aouissi, A. Photocatalytic degradation of some dyes under solar light irradiation using ZnO nanoparticles synthesized from Rosmarinus officinalis extract. Green Chem. Lett. Rev. 2022, 15, 460–473. [Google Scholar] [CrossRef]
- Amini, M.; Ashrafi, M. Photocatalytic degradation of some organic dyes under solar light irradiation using TiO2 and ZnO nanoparticles. Nanochem. Res. 2016, 1, 79–86. [Google Scholar]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 photocatalysis: Concepts, mechanisms, and challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef]
- Yu, B.; Leung, K.M.; Guo, Q.; Lau, W.M.; Yang, J. Synthesis of Ag–TiO2 composite nano thin film for antimicrobial application. Nanotechnology 2011, 22, 115603. [Google Scholar] [CrossRef]
- Nutescu Duduman, C.; Gómez de Castro, C.; Apostolescu, G.A.; Ciobanu, G.; Lutic, D.; Favier, L.; Harja, M. Enhancing the TiO2-Ag Photocatalytic Efficiency by Acetone in the Dye Removal from Wastewater. Water 2022, 14, 2711. [Google Scholar] [CrossRef]
- Hashim, F.S.; Alkaim, A.F.; Mahdi, S.M.; Alkhayatt, A.H.O. Photocatalytic degradation of GRL dye from aqueous solutions in the presence of ZnO/Fe2O3 nanocomposites. Compos. Commun. 2019, 16, 111–116. [Google Scholar] [CrossRef]
- Akpan, U.G.; Hameed, B.H. Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: A review. J. Hazard. Mater. 2009, 170, 520–529. [Google Scholar] [CrossRef]
- Abass, K.H.; Shinen, M.H.; Alkaim, A.F. Preparation of TiO2 nanolayers via sol-gel method and study the optoelectronic properties assolar cell applications. J. Eng. Appl. Sci. 2018, 13, 9631–9637. [Google Scholar]
- Wahyuni, E.T.; Roto, R.; PrameSwari, M. TiO2/Ag-nanoparticle as a Photocatalyst for Dyes Degradation. In Proceedings of the 15th International Conference on Environmental Science and Technology, Rhodes, Greece, 31 August–2 September 2017. [Google Scholar]
- Pant, B.; Saud, P.S.; Park, M.; Park, S.J.; Kim, H.Y. General one-pot strategy to prepare Ag–TiO2 decorated reduced graphene oxide nanocomposites for chemical and biological disinfectant. J. Alloys Compd. 2016, 671, 51–59. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, F.; Li, Y.; Zhang, D.; Chen, Y. One-step synthesis of Ag@ TiO2 nanoparticles for enhanced photocatalytic performance. Nanomaterials 2018, 8, 1032. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cha, J.K.; Fu, G.; Cho, E.J.; Kim, H.S.; Kim, S.H. Aerosol processing of Ag/TiO2 composite nanoparticles for enhanced photocatalytic water treatment under UV and visible light irradiation. Ceram. Int. 2022, 48, 9434–9441. [Google Scholar] [CrossRef]
- Hashim, F.S.; Alkaim, A.F.; Salim, S.J.; Alkhayatt, A.H.O. Effect of (Ag, Pd) doping on structural, and optical properties of ZnO nanoparticales: As a model of photocatalytic activity for water pollution treatment. Chem. Phys. Lett. 2019, 737, 136828. [Google Scholar] [CrossRef]
- Ahmed, L.M.; Alkaim, A.F.; Halbus, A.F.; Hussein, F.H. Photocatalytic hydrogen production from aqueous methanol solution over metallized TiO2. Int. J. ChemTech Res. 2016, 9, 90–98. [Google Scholar]
- Aljeboree, A.M.; Alkaim, A.F. Comparative removal of three textile dyes from aqueous solutions by adsorption: As a model (corn-cob source waste) of plants role in environmental enhancement. Plant Arch. 2019, 19, 1613–1620. [Google Scholar]
- Sass, D.T.; Massima Mouele, E.S.; Ross, N. Nano Silver-Iron-Reduced Graphene Oxide Modified Titanium Dioxide Photocatalytic Remediation System for Organic Dye. Environments 2019, 6, 106. [Google Scholar] [CrossRef]
- Ravishankar, T.N.; Ramakrishnappa, T.; Nagabhushana, H.; Souza, V.S.; Dupont, J.; Nagaraju, G. Hydrogen generation and degradation of trypan blue using fern-like structured silver-doped TiO2 nanoparticles. New J. Chem. 2015, 39, 1421–1429. [Google Scholar] [CrossRef]
- Zhang, M.; Jiang, S.; Han, F.; Chen, H.; Wang, N.; Liu, L.; Liu, L. Recyclable Ag/TiO2@ PDMS Coated Cotton Fabric with Visible-Light Photocatalytic for Efficient Water Purification. Res. Sq. 2021. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).