Influence of Multiwalled Carbon Nanotubes in Sulfur/Carbon Nanotube Composites Synthesized Using Solution Casting Method †
Abstract
:1. Introduction
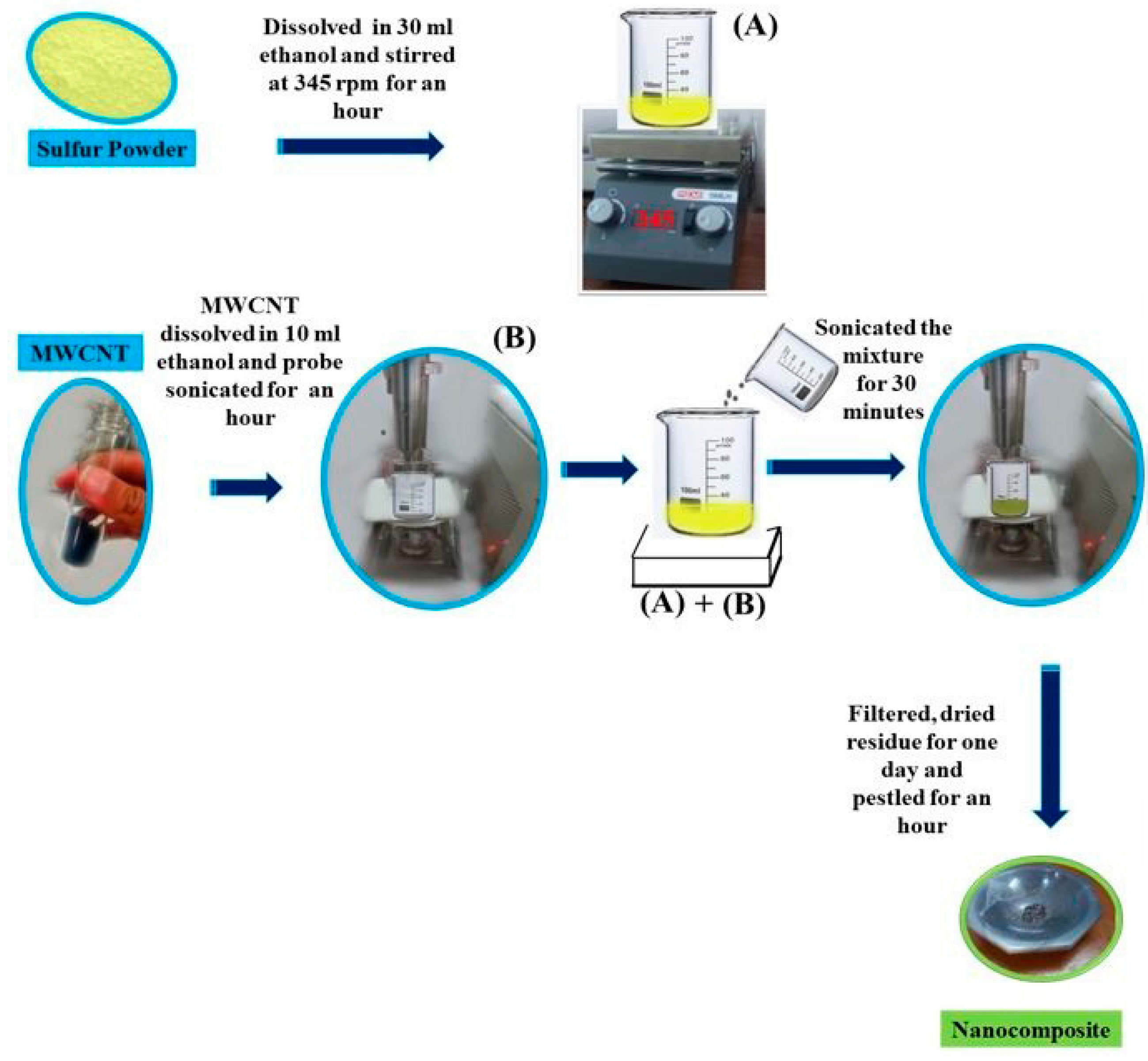
2. Materials and Method
2.1. Materials
2.2. Characterization
3. Results and Discussion
3.1. XRD Diffractogram Studies
3.2. Morphological Studies
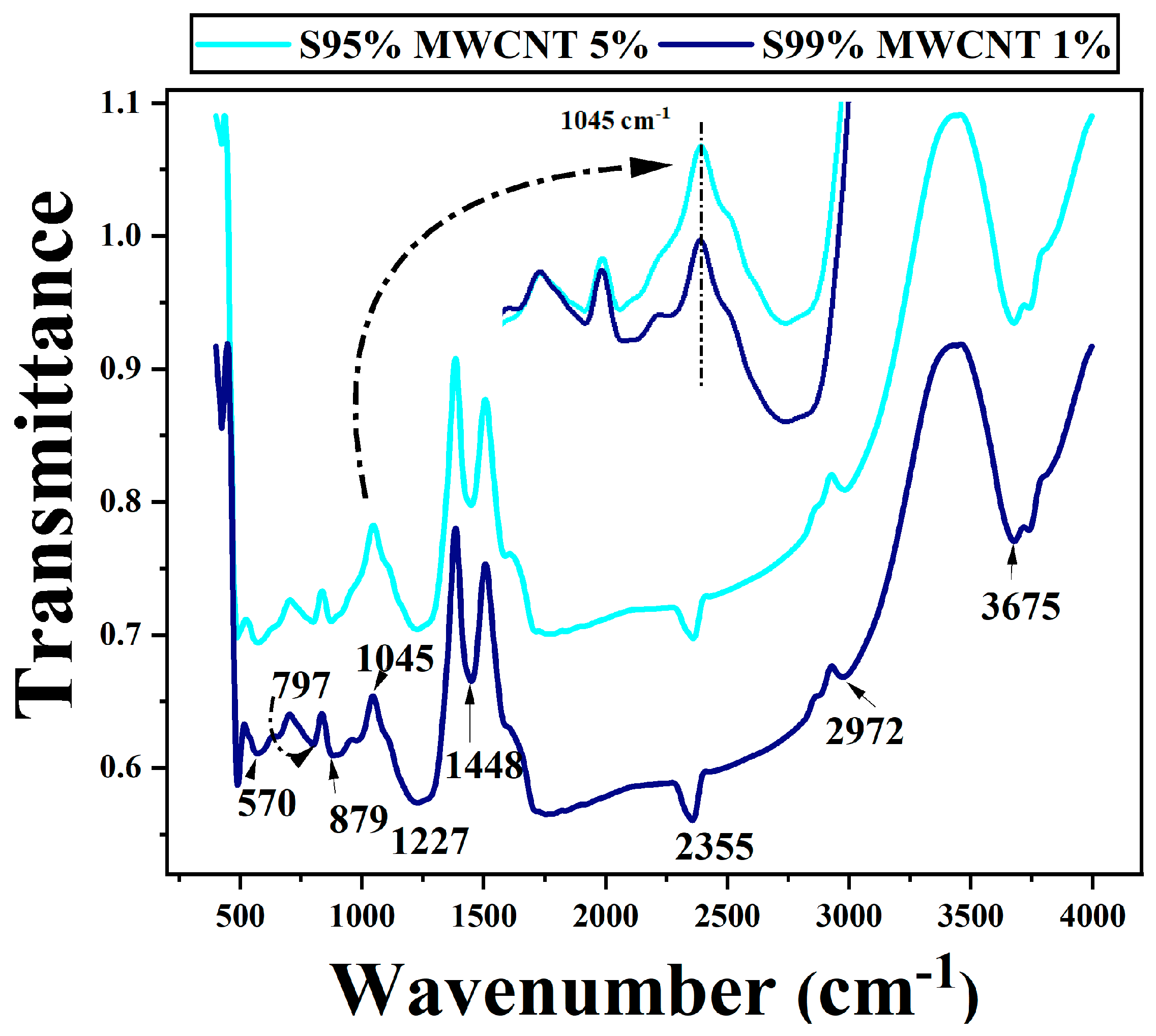
3.3. FTIR Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deb, A.K.S.; Dhume, N.; Dasgupta, K.; Ali, S.M.; Shenoy, K.T.; Mohan, S. Sulfur Ligand Functionalized Carbon Nanotubes for Removal of mercury from wastewater–experimental and density functional theoretical study. Sep. Sci. Technol. 2019, 54, 1573–1587. [Google Scholar] [CrossRef]
- Raphael, J.; Bhat, A.G.; Siby, J.; Geevarghese, B.D.; George, N.; Chacko, T. Mechanical buckling of nanocomposites: Experimental and numerical investigations. Polym. Polym. Compos. 2021, 29, 1313–1324. [Google Scholar] [CrossRef]
- Minchitha, K.U.; Rekha, M.; Nagaraju, N.; Kathyayini, N. Evaluation of Catalytic Activity of Acid Activated Multiwalled Carbon Nanotubes in an Esterification Reaction. Curr. Catal. 2014, 4, 20–30. [Google Scholar] [CrossRef]
- Bhatt, A.; Jain, A.; Gurnany, E.; Jain, R.; Modi, A.; Jain, A. Carrier for Drug Delivery; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 465–501. [Google Scholar]
- Raza, I.; Hussain, M.; Khan, A.N.; Katzwinkel, T.; Feldhusen, J. Properties of Light Weight Multi Walled Carbon Nano Tubes (MWCNTs) Nano- Composites. Int. J. Light. Mater. Manuf. 2020, 4, 195–202. [Google Scholar] [CrossRef]
- Can, A.; Kaya, F.; Kaya, C. A study on optimum surfactant to multiwalled carbon nanotube ratio in alcoholic stable suspensions via UV—Vis absorption spectroscopy and zeta potential analysis. Ceram. Int. 2020, 46, 29120–29129. [Google Scholar] [CrossRef]
- Fujimori, T.; Morelos-Gómez, A.; Zhu, Z.; Muramatsu, H.; Futamura, R.; Urita, K.; Terrones, M.; Hayashi, T.; Endo, M.; Young Hong, S.; et al. Conducting linear chains of sulfur inside carbon nanotubes. Nat. Commun. 2013, 4, 2162. [Google Scholar] [CrossRef] [PubMed]
- Aftab, J.; Mehmood, S.; Ali, A.; Ahmad, I.; Bhopal, M.F.; Rehman, M.Z.U.; Shah, M.Z.U.; Shah, A.U.; Wang, M.; Khan, M.F.; et al. Synergetic Electrochemical Performance of Tungsten Oxide/Tungsten Disulfide/MWCNTs for High-Performance Aqueous Asymmetric Supercapattery Devices. J. Alloys Compd. 2023, 965, 171366. [Google Scholar] [CrossRef]
- Isacfranklin, M.; Yuvakkumar, R.; Kungumadevi, L.; Ravi, G.; Rajendran, V. Boron, Nitrogen, Sulfur Heteroatom Influence Effect on Direct Growth Carbon Nanotubes on Ni Foam for Anode Electrodes. Electrochim. Acta 2023, 468, 142961. [Google Scholar] [CrossRef]
- Stuart, B.H. Infrared Spectroscopy: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar] [CrossRef]
- Rasheed, H.K.; Kareem, A.A. Effect of Multiwalled Carbon Nanotube Reinforcement on the Opto-Electronic Properties of Polyaniline/c-Si Heterojunction. J. Opt. Commun. 2021, 42, 25–29. [Google Scholar] [CrossRef]
- Infrared Spectroscopy. Available online: https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/spectrpy/infrared/infrared.htm (accessed on 2 July 2023).
- Shuit, S.H.; Tan, S.H. Feasibility study of various sulphonation methods for transforming carbon nanotubes into catalysts for the esterification of palm fatty acid distillate. Energy Convers. Manag. 2014, 88, 1283–1289. [Google Scholar] [CrossRef]
- Patodia, T.; Sharma, K.B.; Dixit, S.; Katyayan, S.; Agarwal, G.; Jain, A.; Jain, S.K.; Tripathi, B. Carbon nanotube-sulfur nanocomposite electrodes for high energy-foldable lithium sulfur battery. Mater. Today Proc. 2019, 42, 1638–1641. [Google Scholar] [CrossRef]
- Jadhav, C.D.; Pandit, B.; Karade, S.S.; Sankapal, B.R.; Chavan, P.G. Enhanced field emission properties of V2O5/MWCNTs nanocomposite. Appl. Phys. A Mater. Sci. Process. 2018, 124, 794. [Google Scholar] [CrossRef]

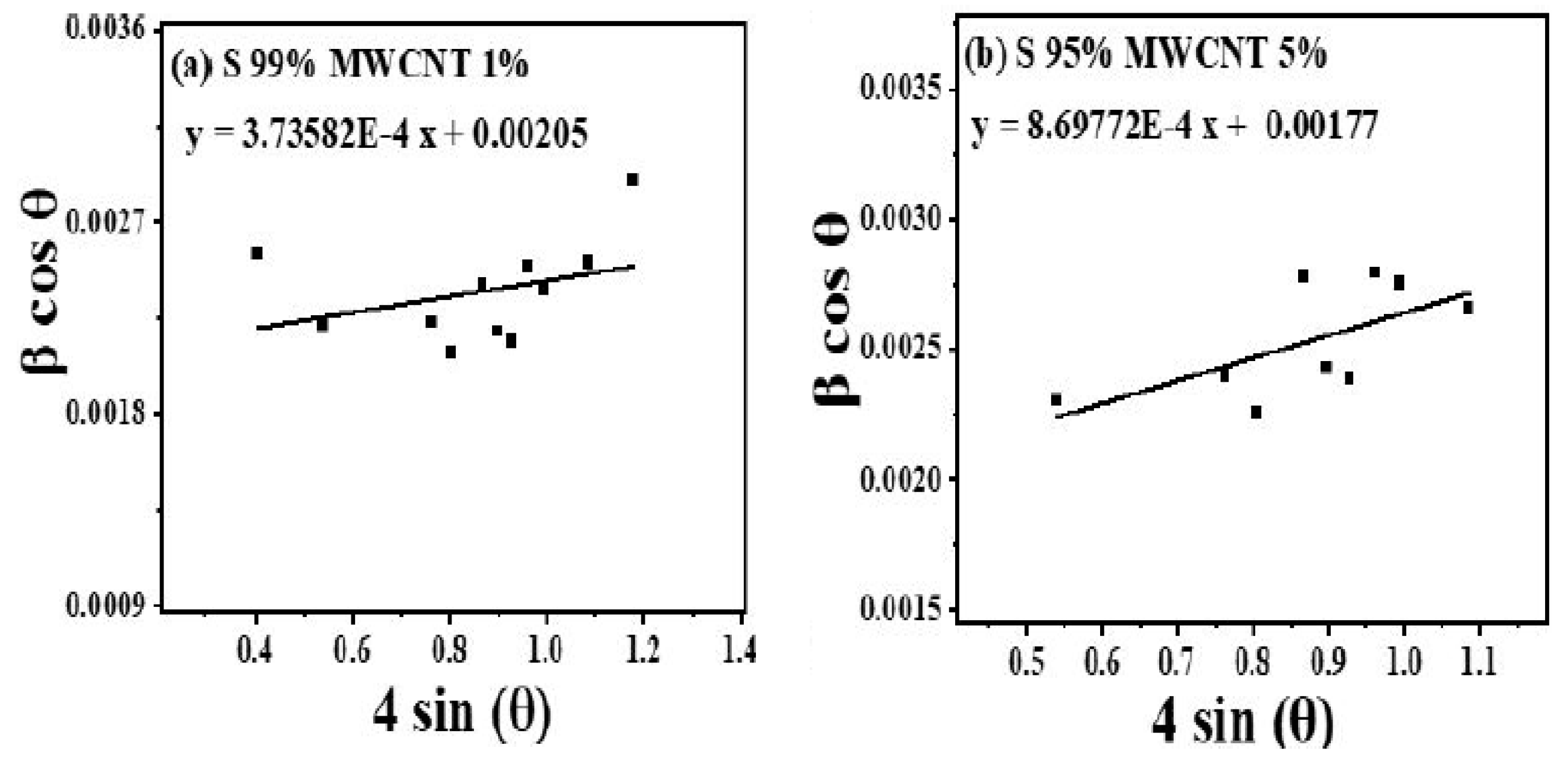



| Sr. No. | Sample Name | Strain (ϵ × 10−4) | Dislocation Density (δ) (10−4 nm−2) | Crystallite Size tDS (in nm) | Crystallite Size tMS (in nm) | Crystallite Size tWH (in nm) |
|---|---|---|---|---|---|---|
| 1. | S99% MWCNT 1% | 3.74 | 2.96 | 58.92 | 64.75 | 67.64 |
| 2. | S95% MWCNT 5% | 8.70 | 3.35 | 55.17 | 64.90 | 78.34 |
| Sr. No. | Position of Bands (cm−1) | Assignment of Bands |
|---|---|---|
| 1 | 570 | C–H out-of-plane bending, =C–H out-of-plane bending in alkenes |
| 2 | 797 | C–H out-of-plane bending of aromatic compounds, =C–H out-of-plane bending in alkenes [12] |
| 3 | 879 | S-OR ester group [12] |
| 4 | 1045 | C-O stretching band of alcohols, S=O stretching of sulfur compounds [13] |
| 5 | 1227 | Aliphatic C-O stretching of esters |
| 6 | 1448 | C=C stretching of carboxylic acid [14] |
| 7 | 2355 | Combination C-H stretching [15] |
| 8 | 2972 | C-H stretching of alkane [14] |
| 9 | 3675 | O-H stretching of alcohol [15] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jain, K.; Jain, S.K.; Malhotra, A.; Dixit, S.; Tripathi, B.; Sahu, R. Influence of Multiwalled Carbon Nanotubes in Sulfur/Carbon Nanotube Composites Synthesized Using Solution Casting Method. Eng. Proc. 2023, 59, 217. https://doi.org/10.3390/engproc2023059217
Jain K, Jain SK, Malhotra A, Dixit S, Tripathi B, Sahu R. Influence of Multiwalled Carbon Nanotubes in Sulfur/Carbon Nanotube Composites Synthesized Using Solution Casting Method. Engineering Proceedings. 2023; 59(1):217. https://doi.org/10.3390/engproc2023059217
Chicago/Turabian StyleJain, Karishma, Sushil Kumar Jain, Anu Malhotra, Shalini Dixit, Balram Tripathi, and Rajesh Sahu. 2023. "Influence of Multiwalled Carbon Nanotubes in Sulfur/Carbon Nanotube Composites Synthesized Using Solution Casting Method" Engineering Proceedings 59, no. 1: 217. https://doi.org/10.3390/engproc2023059217






