Optimization of Extracted Phenolic Compounds from Oregano through Accelerated Solvent Extraction Using Response Surface Methodology †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Accelerated Solvent Extraction (ASE)
2.2. Experimental Design
2.3. Analyses
2.4. Statistical Analysis
3. Results and Discussion
3.1. Statistical Analysis and Model Fitting
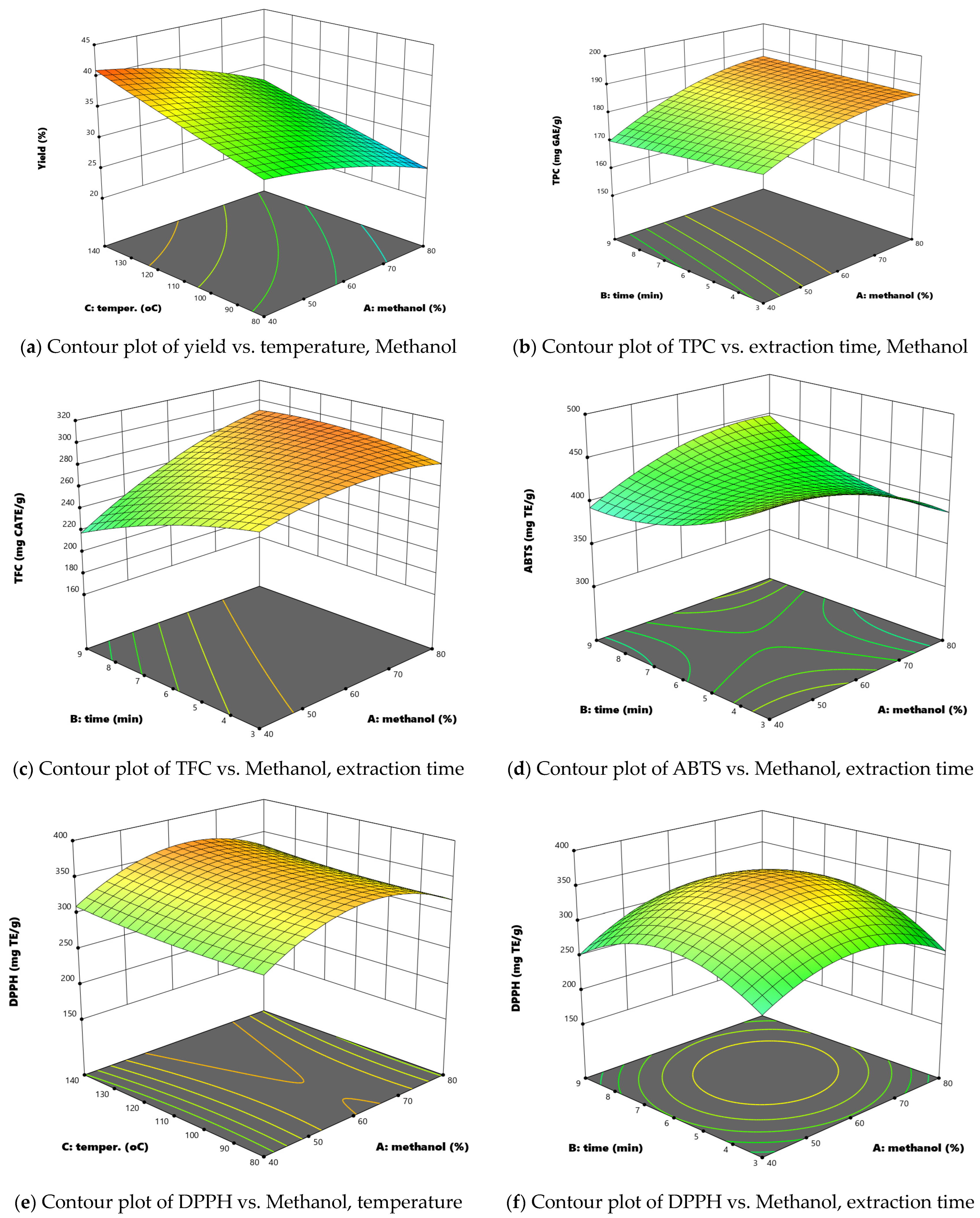
3.2. Influence of Independent Factors on the Investigated Responses
3.3. Optimization Study
3.4. Identification and Quantification of Phenolic Compounds by LC–DAD-MS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saha, S.; Walia, S.; Kundu, A.; Sharma, K.; Paul, R.K. Optimal extraction and fingerprinting of carotenoids by accelerated solvent extraction and liquid chromatography with tandem mass spectrometry. Food Chem. 2015, 177, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Kellogg, J.J.; Wallace, E.D.; Graf, T.N.; Oberlies, N.H.; Cech, N.B. Conventional and accelerated-solvent extractions of green tea (Camellia sinensis) for metabolomics-based chemometrics. J. Pharm. Biomed. Anal. 2017, 145, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Irakli, M.; Skendi, A.; Bouloumpasi, E.; Christaki, S.; Biliaderis, C.G.; Chatzopoulou, P. Sustainable recovery of phenolic compounds from distilled rosemary by-product using green extraction methods: Optimization, comparison, and antioxidant activity. Molecules 2023, 28, 6669. [Google Scholar] [CrossRef] [PubMed]
- Chamali, S.; Bendaoud, H.; Bouajila, J.; Camy, S.; Saadaoui, E.; Condoret, J.S.; Romdhane, M. Optimization of accelerated solvent extraction of bioactive compounds from Eucalyptus intertexta using response surface methodology and evaluation of its phenolic composition and biological activities. J. Appl. Res. Med. Aromat. Plants 2023, 35, 100464. [Google Scholar] [CrossRef]
- Pearson, C.H.; Cornish, K.; McMahan, C.M.; Rath, D.J.; Whalen, M. Natural rubber quantification in sunflower using an automated solvent extractor. Ind. Crop. Prod. 2010, 31, 469–475. [Google Scholar] [CrossRef]
- Gomes, S.V.; Portugal, L.A.; dos Anjos, J.P.; de Jesus, O.N.; de Oliveira, E.J.; David, J.P.; David, J.M. Accelerated solvent extraction of phenolic compounds exploiting a Box-Behnken design and quantification of five flavonoids by HPLC-DAD in Passiflora species. Microchem. J. 2017, 132, 28–35. [Google Scholar] [CrossRef]
- Şahin, F.; Güllüce, M.; Daferera, D.; Sökmen, A.; Sökmen, M.; Polissiou, M.; Agar, G.; Özer, H. Biological activities of the essential oils and methanol extract of Origanum vulgare ssp. vulgare in the Eastern Anatolia region of Turkey. Food Control 2004, 15, 549–557. [Google Scholar]
- Aligiannis, N.; Kalpoutzakis, E.; Mitaku, S.; Chinou, I.B. Composition and antimicrobial activity of the essential oils of two Origanum species. J. Agric. Food Chem. 2001, 49, 4168–4170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Guo, Y.S.; Wang, C.H.; Li, G.Q.; Xu, J.J.; Chung, H.Y.; Ye, W.C.; Li, Y.L.; Wang, G.C. Phenolic compounds from Origanum vulgare and their antioxidant and antiviral activities. Food Chem. 2014, 152, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.H.; Chan, L.P.; Ding, H.Y.; So, E.C.; Lin, R.J.; Wang, H.M.; Chen, Y.-G.; Chou, T.H. Free radical scavenging activity of 4-(3,4-dihydroxybenzoyloxymethyl) phenyl-O-β-D-glucopyranoside from Origanum vulgare and its protection against oxidative damage. J. Agric. Food Chem. 2012, 60, 7690–7696. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.Y.; Chou, T.H.; Liang, C.H. Antioxidant and antimelanogenic properties of rosmarinic acid methyl ester from Origanum vulgare. Food Chem. 2010, 123, 254–262. [Google Scholar] [CrossRef]
- Sivropoulou, A.; Papanikolaou, E.; Nikolaou, C.; Kokkini, S.; Lanaras, T.; Arsenakis, M. Antimicrobial and cytotoxic activities of Origanum essential oils. J. Agric. Food Chem. 1996, 44, 1202–1205. [Google Scholar] [CrossRef]
- Cvetanović, A.; Švarc-Gajić, J.; Mašković, P.; Savić, S.; Nikolić, L. Antioxidant and biological activity of chamomile extracts obtained by different techniques: Perspective of using superheated water for isolation of biologically active compounds. Ind. Crop. Prod. 2015, 65, 582–591. [Google Scholar] [CrossRef]
- Irakli, M.; Bouloumpasi, E.; Christaki, S.; Skendi, A.; Chatzopoulou, P. Modeling and Optimization of Phenolic Compounds from Sage (Salvia fruticosa L.) Post-Distillation Residues: Ultrasound- versus Microwave-Assisted Extraction. Antioxidants 2023, 12, 549. [Google Scholar] [CrossRef] [PubMed]
- Irakli, M.; Skendi, A.; Bouloumpasi, E.; Chatzopoulou, P.; Biliaderis, C.G. LC-MS Identification and Quantification of Phenolic Compounds in Solid Residues from the Essential Oil Industry. Antioxidants 2021, 10, 2016. [Google Scholar] [CrossRef] [PubMed]
- Freedman, D.A.; Freedman, D.A. A note on screening regression equations. Am. Stat. 1983, 37, 152–155. [Google Scholar] [CrossRef]
- Chew, K.K.; Khoo, M.Z.; Ng, S.Y.; Thoo, Y.Y.; Aida, W.W.; Ho, C.W. Effect of ethanol concentration, extraction time and extraction temperature on the recovery of phenolic compounds and antioxidant capacity of Orthosiphon stamineus extracts. Int. Food Res. J. 2011, 18, 1427. [Google Scholar]
- Liang, X.; Nielsen, N.J.; Christensen, J.H. Selective pressurized liquid extraction of plant secondary metabolites: Convallaria majalis L. as a case. Anal. Chim. Acta X 2020, 4, 100040. [Google Scholar] [CrossRef] [PubMed]
- Chun, S.S.; Vattem, D.A.; Lin, Y.T.; Shetty, K. Phenolic antioxidants from clonal oregano (Origanum vulgare) with antimicrobial activity against Helicobacter pylori. Process Biochem. 2005, 40, 809–816. [Google Scholar] [CrossRef]
| Independent Variables | Levels | ||
|---|---|---|---|
| (−1) | (0) | (+1) | |
| X1—solvent concentration (% v/v) | 40 | 60 | 80 |
| X2—static extraction time (min) | 3 | 6 | 9 |
| X3—extraction temperature (°C) | 80 | 110 | 140 |
| Regression Equation in Uncoded Units | ||
|---|---|---|
| Extraction Yield | = | 33.70 − 3.98 X1 + 4.00 X3 |
| TPC | = | 183.96 + 6.93 X1 − 3.93 X12 + 6.43 X1 × X3 |
| TFC | = | 282.47 + 20.17 X1 − 11.67 X2 + 5.98 X1 × X2 + 25.63 X1 × X3 − 12.05 X32 |
| ABTS | = | 30.81 X1 × X2 + 26.94 X1 × X3 |
| DPPH | = | 352.58 + 41.20 X12 − 46.51 X22 |
| Term | Yield | TPC | TFC | ABTS | DPPH | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | |
| Model | 13.65 | 0.0002 | 5.56 | 0.0065 | 6.15 | 0.0045 | 2.64 | 0.0732 | 3.66 | 0.0276 |
| X1 | 56.86 | <0.0001 | 22.60 | 0.0008 | 16.44 | 0.0023 | 0.1519 | 0.7049 | 0.6710 | 0.4318 |
| X2 | 1.45 | 0.2565 | 0.8505 | 0.3781 | 5.50 | 0.0410 | 0.0089 | 0.9268 | 0.3136 | 0.5878 |
| X3 | 57.33 | <0.0001 | 0.2658 | 0.6174 | 1.47 | 0.2532 | 1.84 | 0.2045 | 0.1643 | 0.6938 |
| X1X2 | 0.0700 | 0.7968 | 0.8368 | 0.3818 | 5.98 | 0.0345 | 6.50 | 0.0288 | 0.4281 | 0.5277 |
| X1X3 | 0.1393 | 0.7168 | 11.40 | 0.0071 | 15.53 | 0.0028 | 4.97 | 0.0498 | 0.0025 | 0.9609 |
| X2X3 | 1.58 | 0.2372 | 4.35 | 0.0636 | 0.0505 | 0.8267 | 0.8691 | 0.3732 | 1.63 | 0.2311 |
| X12 | 4.05 | 0.0720 | 7.69 | 0.0197 | 4.29 | 0.0653 | 4.11 | 0.0702 | 13.77 | 0.0040 |
| X22 | 0.8753 | 0.3715 | 0.0047 | 0.9467 | 1.76 | 0.2142 | 4.24 | 0.0665 | 17.54 | 0.0019 |
| X32 | 0.0171 | 0.8985 | 2.60 | 0.1377 | 6.18 | 0.0322 | 0.1417 | 0.7145 | 0.1575 | 0.6998 |
| Lack of fit | 2.58 | 0.1610 | 0.7787 | 0.6048 | 2.62 | 0.1568 | 4.64 | 0.0588 | 4.63 | 0.0591 |
| R2 | 0.9247 | 0.8334 | 0.8470 | 0.7038 | 0.7673 | |||||
| R2adj. | 0.8570 | 0.7034 | 0.7093 | 0.4372 | 0.5578 | |||||
| C.V.% * | 5.85 | 3.00 | 6.99 | 8.14 | 14.25 | |||||
| Responses | Predicted | Experimental 1 | % Differences (CV) |
|---|---|---|---|
| Extraction Yield (%) | 33.7 ± 2.0 | 31.44 ± 0.51 | 4.91 |
| TPC (mg GAE/g) | 184.0 ± 5.4 | 183.57 ± 2.47 | 0.16 |
| TFC (mg CATEg) | 282.5 ± 18.4 | 298.54 ± 6.54 | 3.90 |
| ABTS (mg TE/g) | 421.8 ± 34.2 | 473.14 ± 21.14 | 8.11 |
| DPPH (mg TE/g) | 352.6 ± 42.1 | 298.38 ± 5.80 | 11.77 |
| Phenolic Compounds | Mean Value ± Standard Deviation 1 (mg/g Extract) |
|---|---|
| Rosmarinic acid | 173.16 ± 8.16 |
| Salvianolic acid B | 166.24 ± 16.24 |
| Vicenin-2 | 54.01 ± 0.99 |
| Salvianolic acid isomer III | 8.55 ± 0.25 |
| Salvianolic acid isomer II | 8.43 ± 0.31 |
| Salvianolic acid isomer I | 8.05 ± 0.40 |
| Eriodictyol | 6.44 ± 0.08 |
| Carvacrol | 4.40 ± 0.60 |
| Taxifolin | 2.75 ± 0.10 |
| Naringenin | 2.28 ± 0.07 |
| Cryptochlorogenic acid | 1.22 ± 0.03 |
| Luteolin | 1.15 ± 0.12 |
| Caffeic acid | 1.05 ± 0.08 |
| Aromadendrin | 0.82 ± 0.03 |
| Apigenin | 0.65 ± 0.05 |
| Apigenin-7-O-glucoside | 0.56 ± 0.06 |
| Total phenolics | 439.76 ± 23.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panagiotidou, C.; Bouloumpasi, E.; Irakli, M.; Chatzopoulou, P. Optimization of Extracted Phenolic Compounds from Oregano through Accelerated Solvent Extraction Using Response Surface Methodology. Eng. Proc. 2024, 67, 10. https://doi.org/10.3390/engproc2024067010
Panagiotidou C, Bouloumpasi E, Irakli M, Chatzopoulou P. Optimization of Extracted Phenolic Compounds from Oregano through Accelerated Solvent Extraction Using Response Surface Methodology. Engineering Proceedings. 2024; 67(1):10. https://doi.org/10.3390/engproc2024067010
Chicago/Turabian StylePanagiotidou, Christina, Elisavet Bouloumpasi, Maria Irakli, and Paschalina Chatzopoulou. 2024. "Optimization of Extracted Phenolic Compounds from Oregano through Accelerated Solvent Extraction Using Response Surface Methodology" Engineering Proceedings 67, no. 1: 10. https://doi.org/10.3390/engproc2024067010






