Application of Machine Learning for Methanolysis of Waste Cooking Oil Using Kaolinite Geopolymer Heterogeneous Catalyst †
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Esmi, F.; Dalai, A.K.; Hu, Y. Comparison of various machine learning techniques for modeling the heterogeneous acid-catalysed alcoholysis process of biodiesel production from green seed canola oil. Energy Rep. 2024, 12, 321–328. [Google Scholar] [CrossRef]
- Mwenge, P.; Rutto, H.; Enweremadu, C. Biodiesel production using Chlor-alkali brine sludge waste as a heterogeneous catalyst: Optimisation using response surface methodology. Int. J. Sustain. Energy 2022, 41, 832–845. [Google Scholar] [CrossRef]
- Xing, Y.; Zheng, Z.; Sun, Y.; Agha Alikhani, M. A Review on Machine Learning Application in Biodiesel Production Studies. Int. J. Chem. Eng. 2021, 2021, 30. [Google Scholar] [CrossRef]
- Gupta, K.K.; Kalita, K.; Ghadai, R.K.; Ramachandran, M.; Gao, X.-Z. Machine Learning-Based Predictive Modelling of Biodiesel Production—A Comparative Perspective. Energies 2021, 14, 1122. [Google Scholar] [CrossRef]
- Paladino, O.; Neviani, M. Sustainable Biodiesel Production by Transesterification of Waste Cooking Oil and Recycling of Wastewater Rich in Glycerol as a Feed to Microalgae. Sustainability 2021, 14, 273. [Google Scholar] [CrossRef]
- Mwenge, P.; Seodigeng, T. In situ Real-Time Multivariate Analysis of Methanolysis Monitoring of Sunflower Oil Using FTIR. Int. J. Chem. Mol. Eng. 2020, 14, 136–148. [Google Scholar]
- Mwenge, P.; Rutto, H.; Enweremadu, C. Production of Biodiesel using Calcined Brine Sludge Waste from Chor-Alkali Industry as a Heterogeneous Catalyst. Environ. Clim. Technol. 2021, 25, 621–630. [Google Scholar] [CrossRef]
- Saputra, E.; Utama, P.S.; Azis, Y.; Helwani, Z.; Olivia, M.; Widayatno, W.B.; Muraza, O. Geopolymer catalysts derived from palm oil mill ash for biodiesel production from Calophyllum inophyllum oil. Appl. Nanosci. 2022, 12, 3735–3745. [Google Scholar] [CrossRef]
- Botti, R.F.; Innocentini, M.D.M.; Faleiros, T.A.; Mello, M.F.; Flumignan, D.L.; Santos, L.K.; Franchin, G.; Colombo, P. Biodiesel Processing Using Sodium and Potassium Geopolymer Powders as Heterogeneous Catalysts. Molecules 2020, 25, 2839. [Google Scholar] [CrossRef] [PubMed]
- Barbarey, M.S.; El-Sayed Seleman, M.M.; El Kheshen, A.A.; Zawrah, M.F. Utilization of ladle furnace slag for fabrication of geopolymer: Its application as catalyst for biodiesel production. Constr. Build. Mater. 2024, 411, 134226. [Google Scholar] [CrossRef]
- Moayedi, H.; Aghel, B.; Foong, L.K.; Bui, D.T. Feature validity during machine learning paradigms for predicting biodiesel purity. Fuel 2020, 262, 116498. [Google Scholar] [CrossRef]
- Banza, M.; Seodigeng, T. Modelling and Optimisation of Zinc (II) Removal from Synthetic Acid Mine Drainage via Three-Dimensional Adsorbent Using a Machine Learning Approach. In Proceedings of the ECP 2023, Virtual Event, 17–31 May 2023; p. 52. [Google Scholar] [CrossRef]
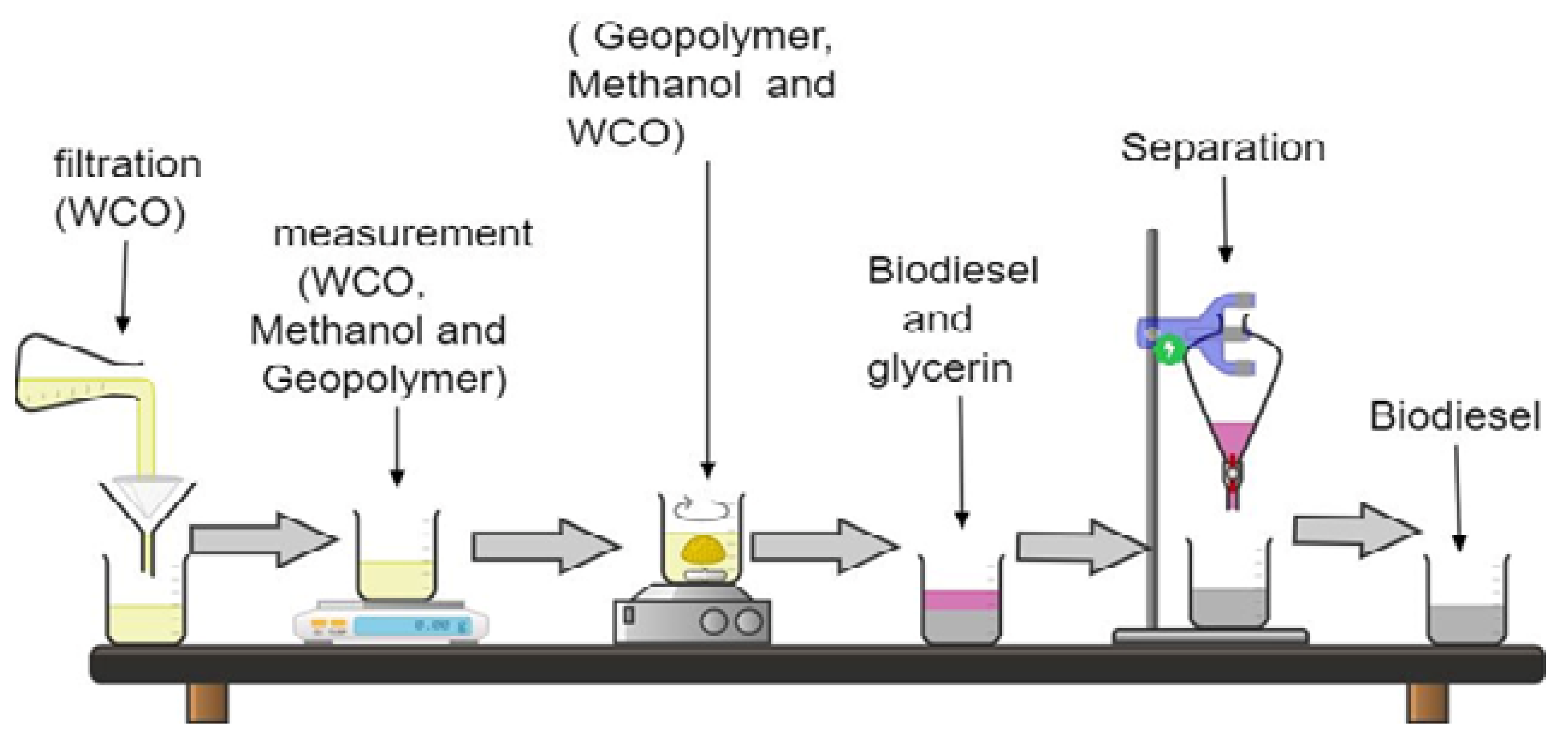
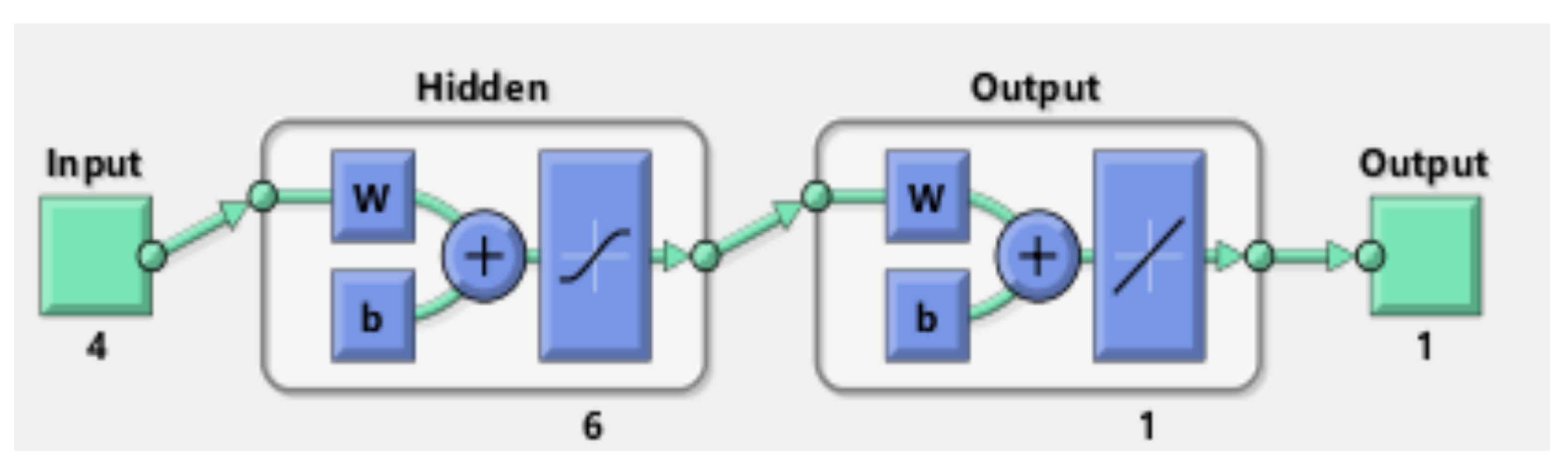
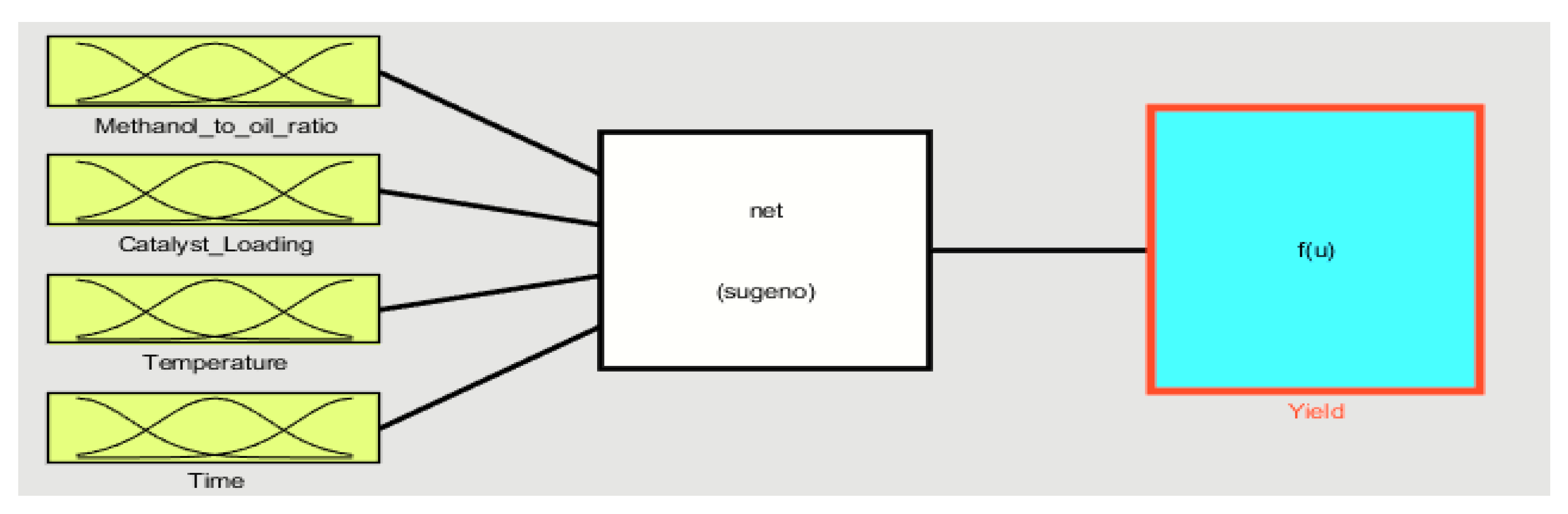

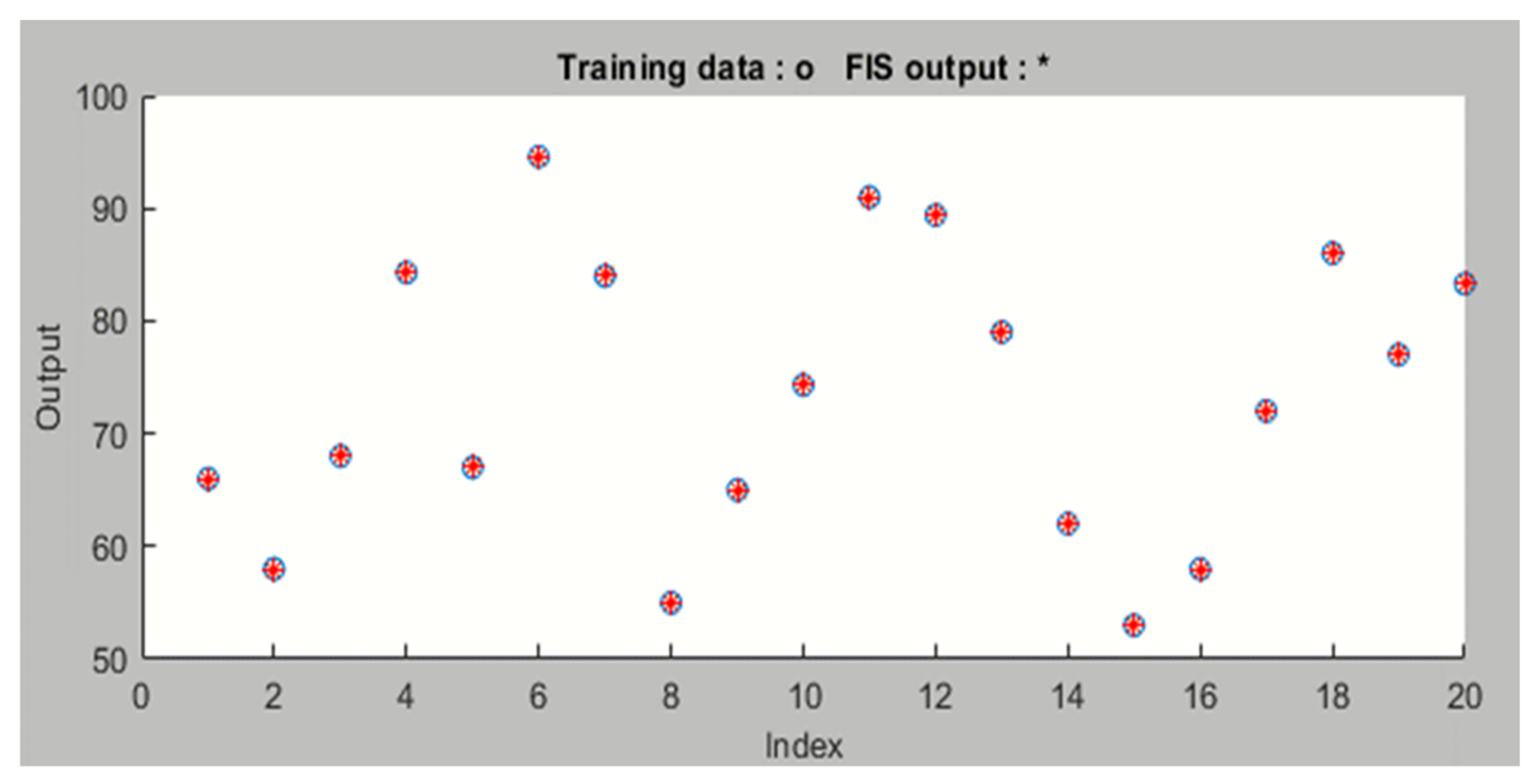

| Input | Range | Output |
|---|---|---|
| Methanol/oil ratio (wt.%) | 10–20 | Yield (%) |
| Catalyst ratio (wt.%) | 2–6 | |
| Temperature (°C) | 60–120 | |
| Time (h) | 2–6 |
| Fatty Acid | Composition (%) |
|---|---|
| Myristic (C14:0) | 0.53 |
| Palmitic (C16:0) | 13.26 |
| Palmitoleic (C16:1) | 1.25 |
| Stearic Acid (C18:0) | 9.57 |
| Oleic Acid (C18:1n9) | 27.12 |
| Linoleic Acid (C18:2n6) | 46.62 |
| Others | 1.65 |
| Error Metrics | RSM | ANN | ANFIS |
|---|---|---|---|
| R2 | 0.9545 | 0.96532 | 0.9695 |
| RMSE | 2.83316 | 2.24452 | 2.13867 |
| MAE | 2.41385 | 1.76933 | 0.74752 |
| MAPE | 2.90697 | 2.3644 | 0.94813 |
| ARE | 0.02907 | 0.02364 | 0.00948 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mwenge, P.; Rutto, H.; Seodigeng, T. Application of Machine Learning for Methanolysis of Waste Cooking Oil Using Kaolinite Geopolymer Heterogeneous Catalyst. Eng. Proc. 2024, 67, 23. https://doi.org/10.3390/engproc2024067023
Mwenge P, Rutto H, Seodigeng T. Application of Machine Learning for Methanolysis of Waste Cooking Oil Using Kaolinite Geopolymer Heterogeneous Catalyst. Engineering Proceedings. 2024; 67(1):23. https://doi.org/10.3390/engproc2024067023
Chicago/Turabian StyleMwenge, Pascal, Hilary Rutto, and Tumisang Seodigeng. 2024. "Application of Machine Learning for Methanolysis of Waste Cooking Oil Using Kaolinite Geopolymer Heterogeneous Catalyst" Engineering Proceedings 67, no. 1: 23. https://doi.org/10.3390/engproc2024067023
APA StyleMwenge, P., Rutto, H., & Seodigeng, T. (2024). Application of Machine Learning for Methanolysis of Waste Cooking Oil Using Kaolinite Geopolymer Heterogeneous Catalyst. Engineering Proceedings, 67(1), 23. https://doi.org/10.3390/engproc2024067023






