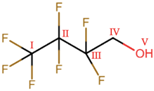
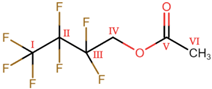
Abstract
As the object of investigation in the present study, reactive distillation based on the transesterification of isopropyl acetate (IPAc) and 2,2,3,3,4,4,4-heptafluorobutanol (HFBol) under acidic conditions is addressed. This process aims to obtain 2,2,3,3,4,4,4-heptafluorobutyl acetate (HFBAc), which is used in the production of non-aqueous electrolytes, ethyllithium sulphate, charge retention medium, ultraviolet light-absorbing oligomers, etc. Through a combination of NMR spectroscopy and GC-MS, it was determined that during the process, the following were primarily formed in the system: target HFBAc and the by-product, isopropanol. The following side-products were identified: di-isopropyl ether, acetic acid, water, and 2,2,3,3,4,4,4-heptafluorobutyl isopropyl ether (HFB-IPEth). No bis(1H,1H-heptafluorobutyl) ether or acetic anhydride were identified in the system. For HFBol, HFBAc and HFB-IPEth the 1H, 19F and 13C{19F}), 19F-19F COSY NMR, and mass spectra were reported in this study.
1. Introduction
As the object of investigation in the present study, the reactive distillation (RD) process based on the transesterification of isopropyl acetate (IPAc) and 2,2,3,3,4,4,4-heptafluorobutanol (HFBol) under acidic conditions is addressed. This process aims at obtain 2,2,3,3,4,4,4-heptafluorobutyl acetate (HFBAc), which is used in pharmaceutical aerosol compositions to reduce particle adhesion to can walls, inhibit particle flocculation, and preventing the creaming of the suspension [1]. It is also used in the production of red-absorbing dyes for imaging and sensing and red-shifted Förster resonance energy transfer (FRET) quencher dyes [2]. It can also be used as a more environmentally friendly analogue of perfluorocarbons for the plasma etching of SiO2 films for semiconductor production [3] and similar processes.
Thus far, information on methods of HFBAc production is practically absent in the literature. A number of sources mention its formation as a side-product during the synthesis of 1,1,1,2,2,3,3-heptafluoro-4-iodobutane (HFBAc ≈ 4%) [4], heptafluorobutyl methacrylate (HFBAc ≈ 1.3%) [5], and a diacetate ester of aldehydrol [6].
Methods for the synthesis of HFBol esters are generally better researched, with well-known methods based on reactions with anhydrides (e.g., isobutyric [7] and methacrylic) [8], halogen anhydrides (e.g., 2-propenoyl chloride) [9], various acids [10,11,12,13,14] (including electrochemical methods at room temperature without the usage of catalysts) [15], methyl and ethyl esters of halogen-substituted acids [16], and methyl methacrylate followed by the polymerization of heptafluorobutyl methacrylate by a double bond [17].
To select suitable conditions for the synthesis and purification of HFBAc, it is necessary to control the composition of the reaction mixture while varying parameters such as time processing, process temperature, and the composition and ratio of initial reagents. At the same time, quantitative analysis is quite difficult without understanding the qualitative composition, including the composition of reaction side-products and their formation conditions. As a number of different side-products are formed during the transesterification reaction between IPAc and HFBol, the aim of the present study is a qualitative analysis of the reaction products via a combination of gas chromatography–mass spectrometry (GC-MS) and nuclear magnetic resonance (NMR) spectroscopy.
2. Materials and Methods
In the present study, the RD process with the initial equimolar ratio of reagents was carried out in batch mode at atmospheric pressure, and the temperature of the reaction varied from 95 to 105 °C. The identification of by-products requires a significant (detectable) amount of the last one in the reaction mixture. Thus, the process was carried out under “harsh” acidic conditions; H2SO4 was used as a catalyst (up to ≈0.2 mol. fr. in the reaction area). Information on the compounds used in this study is presented in Table 1.

Table 1.
Specifications of the compounds used.
Mass spectra were determined using the gas chromatograph Maestro-αMS with a quadrupole mass spectrometer (Interlab, Moscow, Russia). Chromatographic separations were carried out using a capillary column SCI-5MS (30 m × 0.25 mm i.d., film thickness 0.25 μm; MEGA S.r.l, Legnano, Italy). The injector temperature was set at 250 °C in split mode (split ratio 1/100); the column (oven) temperature was 35 °C (4 min). The carrier gas was helium at a constant flow of 1.0 mL·min−1. The ion source temperature and the interface temperature were 230 °C and 250 °C, respectively. The spectra were obtained in SCAN mode. The electron impact ionization energy was 70 eV, and the mass range was m/z 29–300. The Bruker Avance II—300 MHz NMR spectrometer (Bruker Corp., Billerica, MA, USA) was used to obtain 1H and 19F spectra of studied samples at the frequencies of 300.211 MHz and 282.499 MHz, respectively, using internal deuterium lock. The QOne AS400 quantum-I Plus—400 MHz NMR spectrometer (QOneTec, Wuhan, China) was used to obtain 13C{19F}, 19F and 19F-19F COSY spectra of studied samples at the frequencies of 100.549 MHz and 376.263 MHz, respectively, using internal deuterium lock. Tetramethylsilane and triclorofluoromethane were used as external references. Dimethyl sulfoxide-d6 (d-DMSO) was used as a solvent. Mass Comparator MC-1000 (A&D Company Ltd, Tokyo, Japan) was used to measure sample weight.
3. Results and Discussion
The RD process considered in this study is based on the transesterification reaction of IPAc and HFBol under acidic conditions. According to preliminary experimental data, in addition to the two reagents, target product HFBAc and by-product isopropanol (IPol), a number of side-products were found to be present in the reaction mixture. Preliminary studies of the reaction mixture showed the presence of water in the samples. It follows that the presence of alcohols (by-product—IPol and reagent—HFBol) in the system suggests their possible intermolecular dehydration (potentially, the formation of up to three ethers—2,2,3,3,4,4,4-heptafluorobutyl isopropyl ether (HFB-IPEth), di-isopropyl ether (IPEth) and bis(1H,1H-heptafluorobutyl) ether). The presence of water in the reaction area may also lead to a hydration of IPAc to form IPol and acetic acid (AAc).
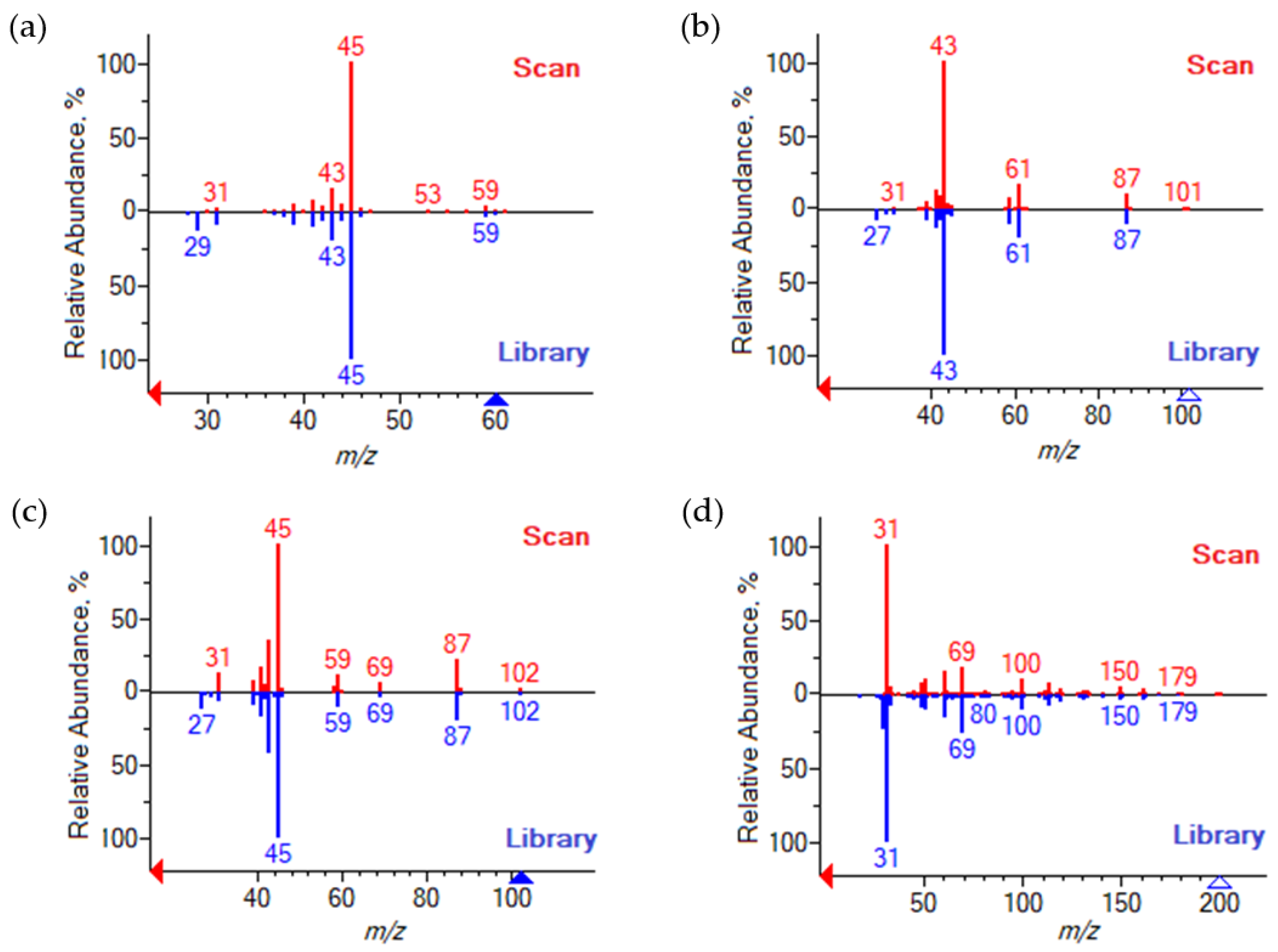
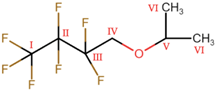
A total of six components were detected in the investigated reaction mixtures using GC-MS, four of which were confirmed using a library search. A comparison of the identified components’ spectra with those from the NIST database is presented in Figure 1. The identified components were IPol (NIST# 289584, ID# 19648, DB: mainlib), IPAc (NIST# 429409, ID# 2939, DB: relib), IPEth (NIST# 423843, ID# 4971, DB: relib), and HFBol (NIST# 133587, ID# 1882 DB: mainlib).
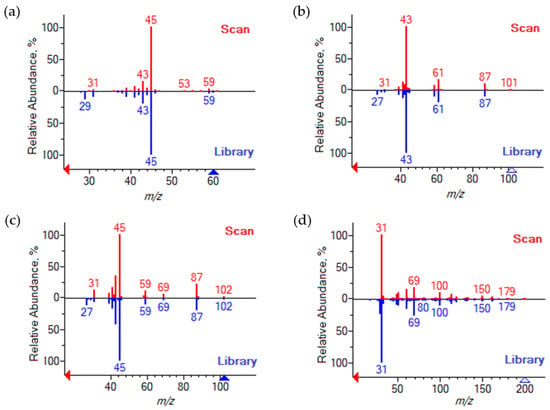
Figure 1.
NIST library spectrum matching (red—experimental spectrum; blue—library spectrum): (a) Isopropanol; (b) Isopropyl acetate; (c) Di-isopropyl ether; (d) 2,2,3,3,4,4,4-heptafluorobutanol.
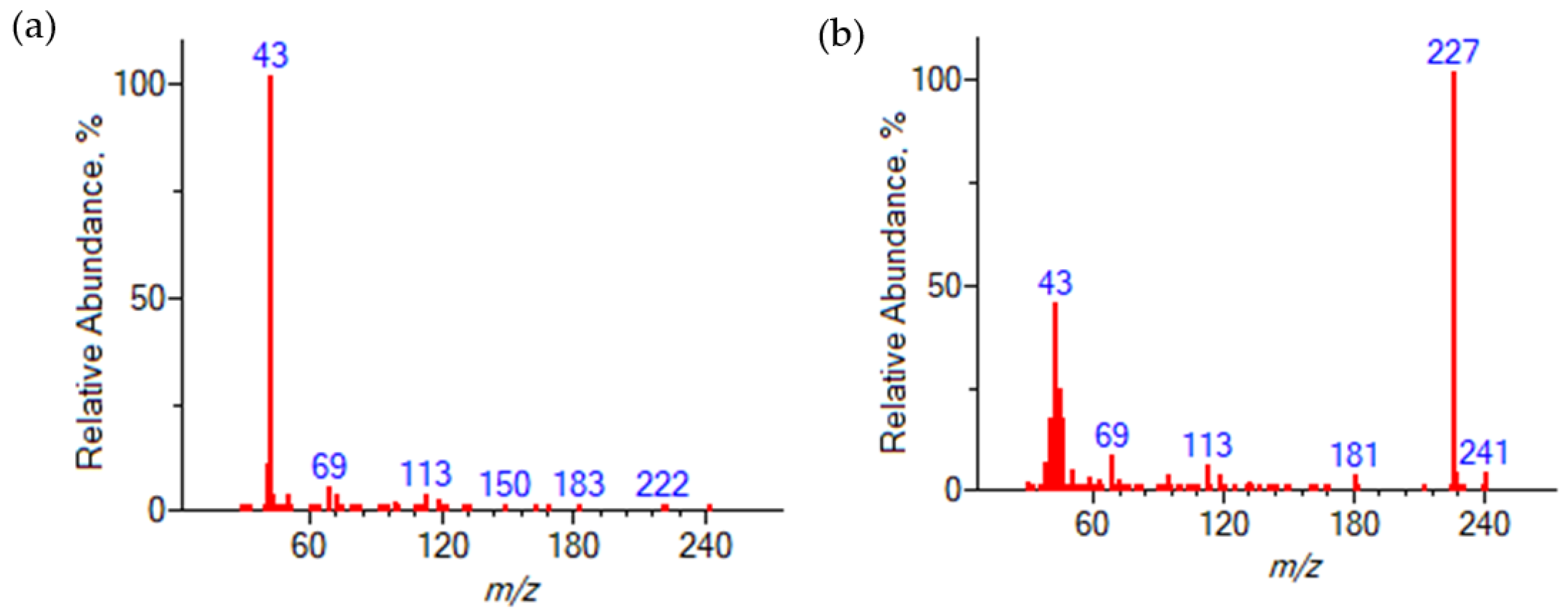
The unknown components were identified by fragment ions obtained by electron ionization (EI). Figure 2 presents the mass spectra of these components. In both cases, the heaviest fragment ion is observed at m/z 242. According to the reaction, two products with this molecular mass are possible, HFBAc and HFB-IPEth. The presence of the 2,2,3,3,4,4,4-heptafluorobutyl fragment is evidenced by the occurrence of fragment ions at m/z 69, 119, 169 and 183, which are consistent with chain fragmentation. The observed difference in base ion can be attributed to the preferred fragmentation pathways. In the case of the HFBAc, the preferred fragmentation pathway results in the loss of the acetate group (m/z 43), while in the case of the HFB-IPEth, the methyl group is eliminated, which results in a base ion at m/z 227. Observed EI fragment mass-to-charge ratios and corresponding products ions for HFBAc and HFB-IPEth are listed in Table 2.
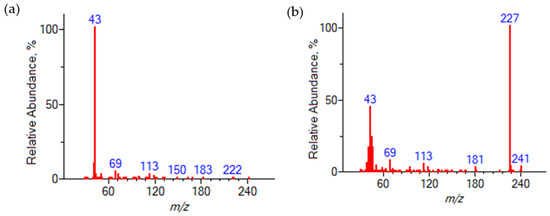
Figure 2.
Mass spectra of 2,2,3,3,4,4,4-heptafluorobutyl acetate (a) and 2,2,3,3,4,4,4-heptafluorobutyl isopropyl ester (b).

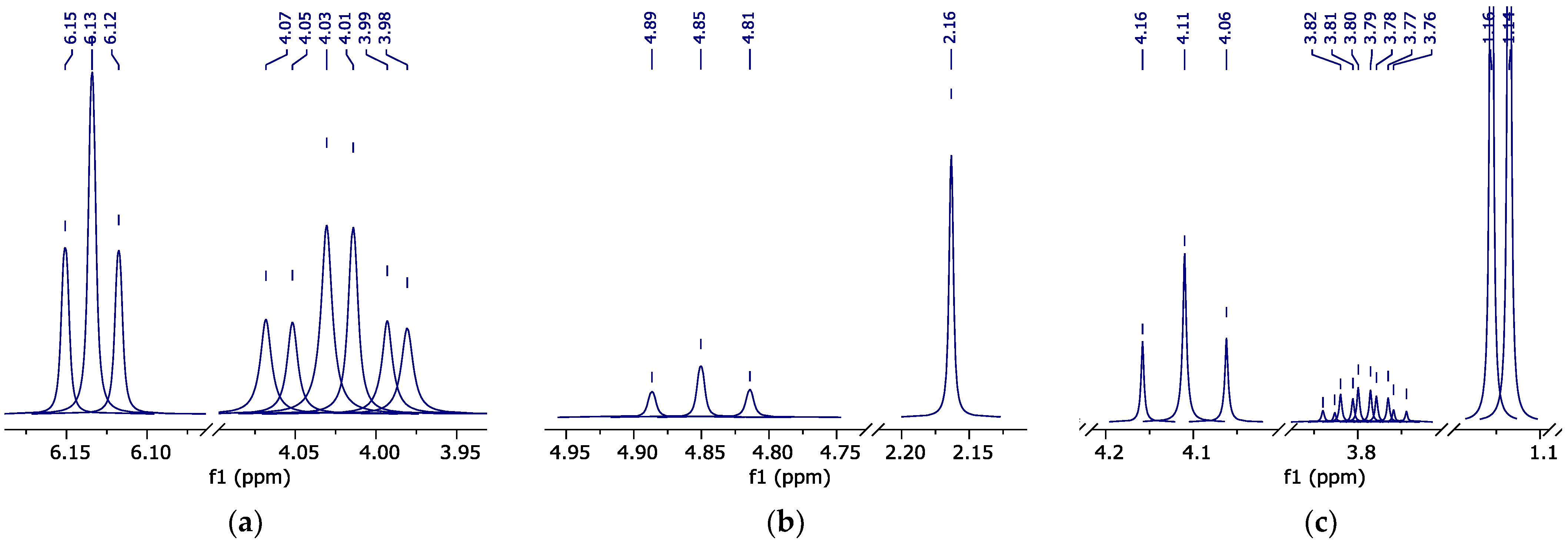
The classical method for organic chemists to identify components is NMR spectroscopy. However, due to the congestion and complexity of the resulting spectra, their interpretation can be unnecessarily time-consuming and sometimes simply impossible. By separating components prior to detection, GC-MS is a powerful tool for the analysis of mixtures of volatile and temperature stable organic compounds, both in addition to NMR spectroscopy and individually. The obtained GC-MS data, literature, and theoretical analyses, as well as NMR spectra of samples of a number of supposed reaction products in pure form provided an opportunity to identify the NMR spectra of the reaction mixture and correlate each of the peaks to their corresponding components (Figure 3).
Figure 3.
1H NMR spectrum of the reaction mixture sample in d-DMSO (peak curves). Red line—water peak (identified by the program as partially deuterated).
Thus, through a combination of NMR spectroscopy and GC-MS, it was determined that during the reaction of IPAc and HFBol under acidic conditions, the following were primarily formed in the system: target HFBAc and by-product IPol. The following side-products were identified: IPEth, AAc, water, and HFB-IPEth. No bis(1H,1H-heptafluorobutyl) ether traces were identified in the system. The overall reaction can be represented as follows:
Therefore, it can be stated that there is an intermolecular dehydration between IPol and HFBol and intermolecular dehydration between IPol molecules, which leads to the appearance of water, HFB-IPEth, and IPEth in the system. The presence of water and AAc in the system indicates that the IPAc hydration is taking place. The esterification reaction of AAc and HFBol, as well as the hydrolysis of HFBAc, can also be stated with full confidence. Among the less likely reactions are the following: HFBol + IPEth ⟷ …; IPAc + IPol ⟷ …; HFBAc + Ipol ⟷ …; HFBAc + IPEth ⟷ …; HFB-IPEth + Aac ⟷ ….
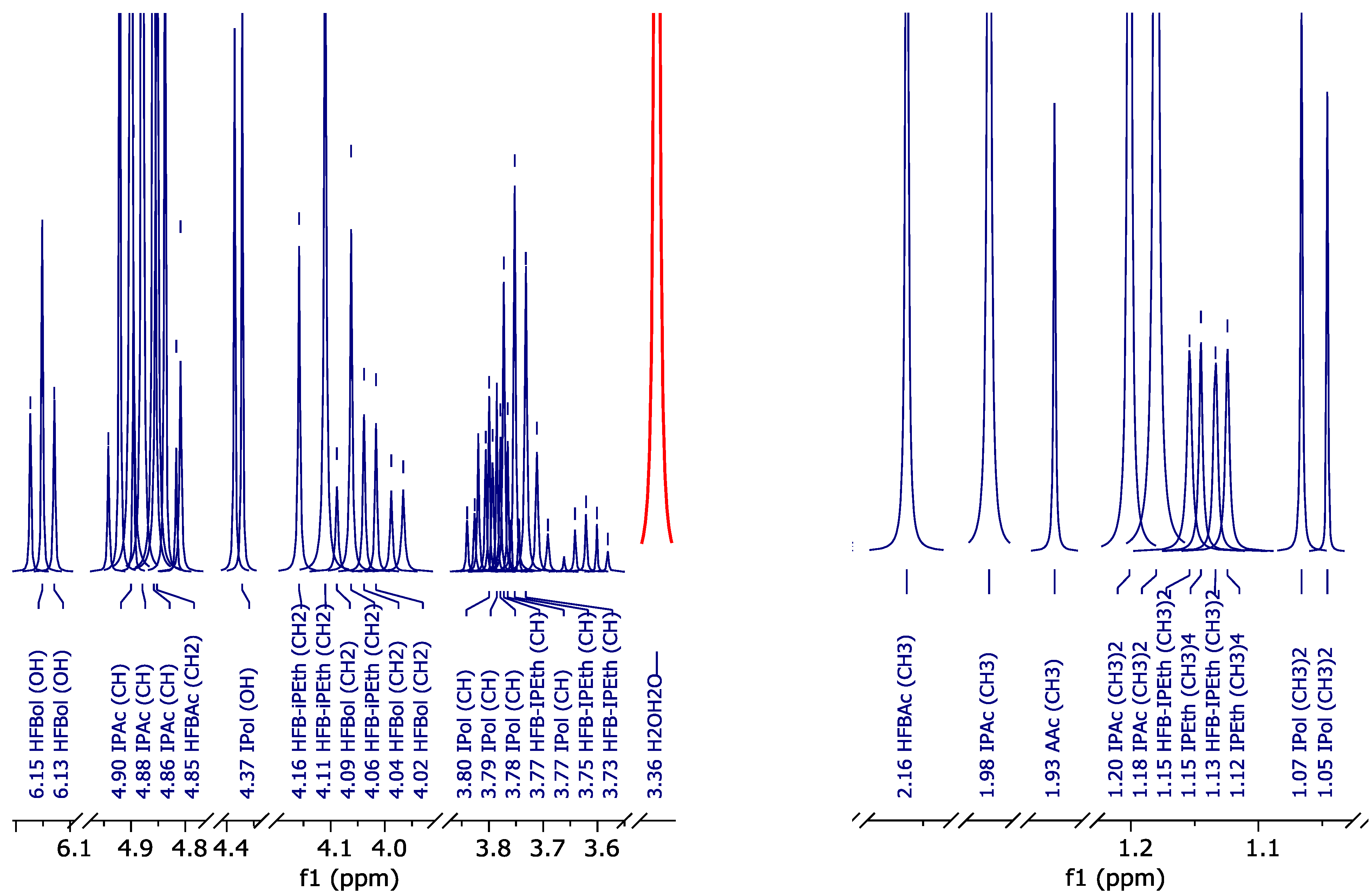
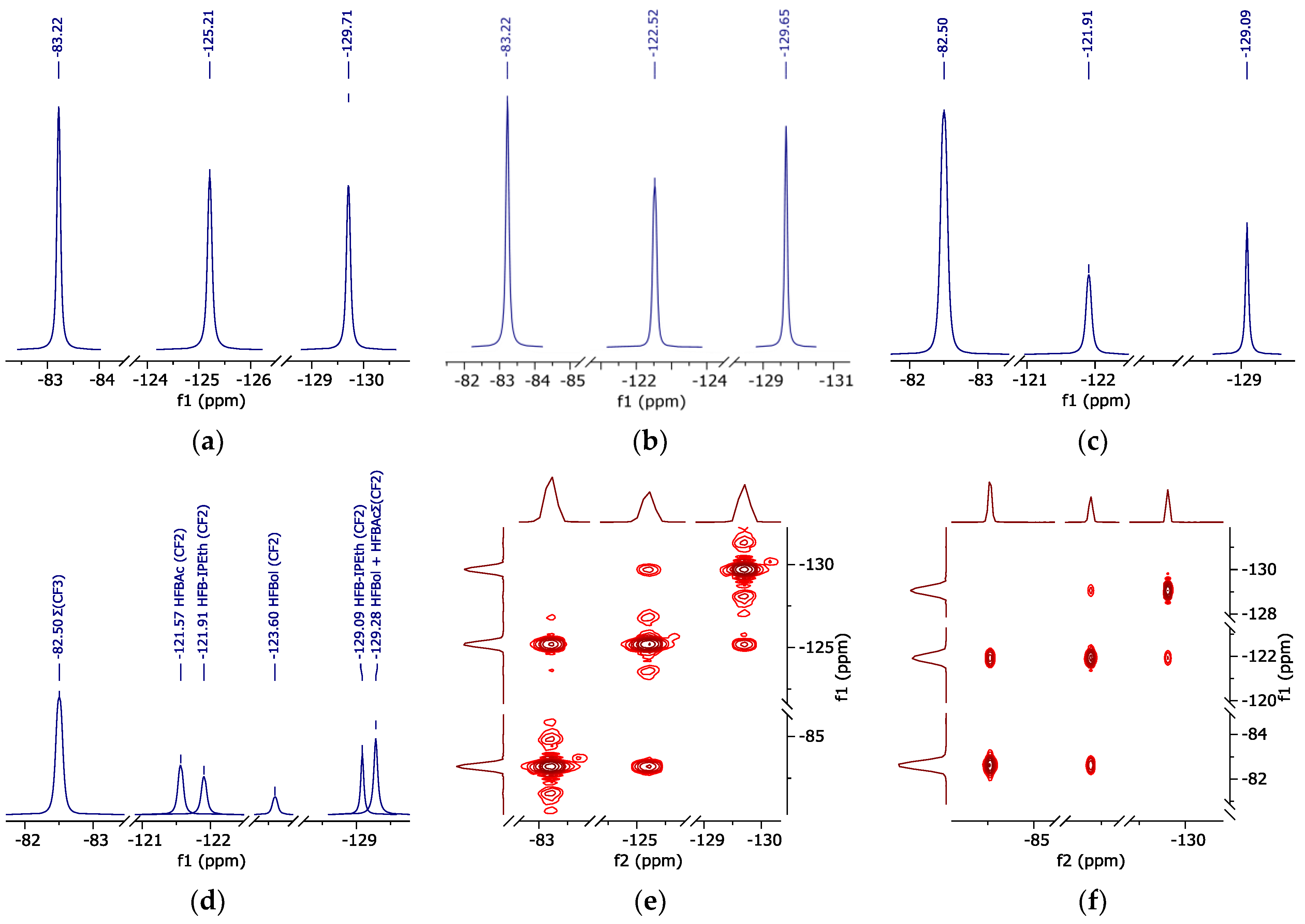
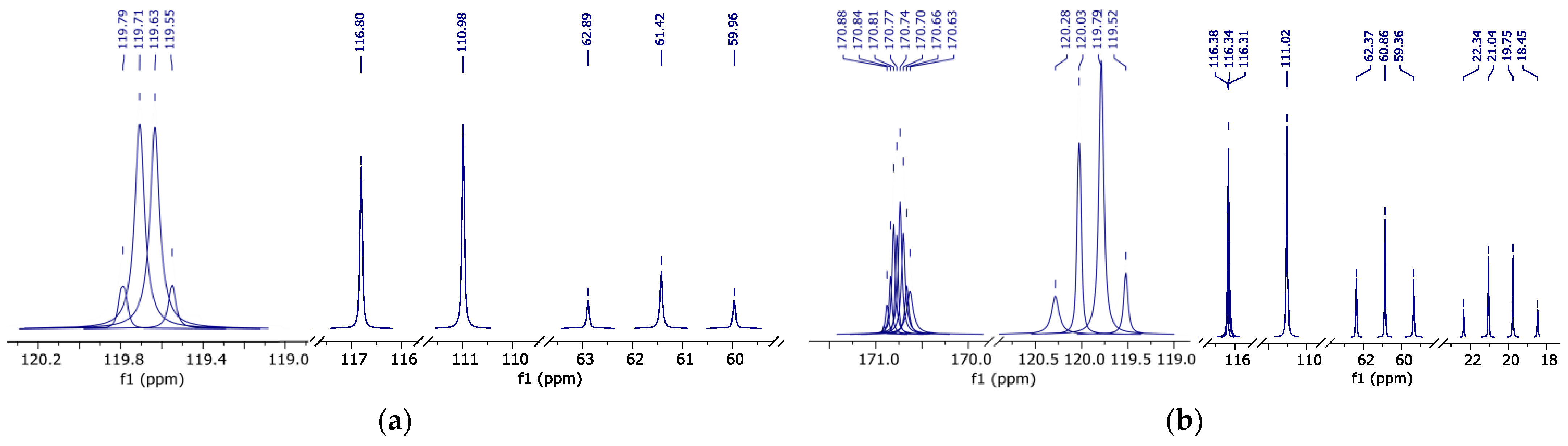
The data obtained allowed us to correlate atom groups of fluorinated compounds with their chemical shifts on 1H in d-DMSO (Figure 4), 19F, 19F-19F COSY (Figure 5) and 13C{19F} (Figure 6) spectra. The data are summarized in Table 3.
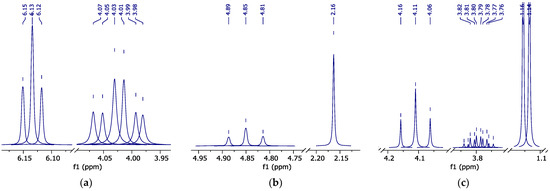
Figure 4.
1H NMR spectrum (peak curves): (a)—pure HFBol in d-DMSO; (b)—pure HFBAc in d-DMSO; and (c)—HFB-iPEth spectrum in d-DMSO isolated from 1H NMR spectrum of the reaction mixture sample.
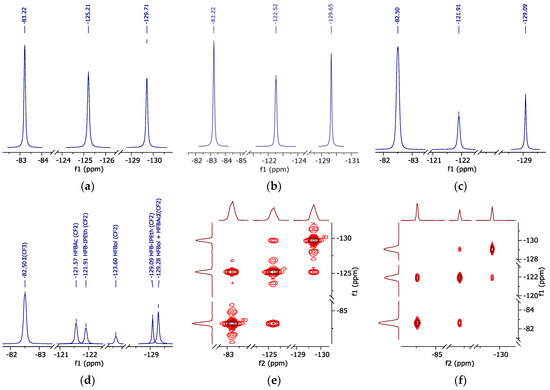
Figure 5.
19F NMR spectrum (peak curves). (a)—pure HFBol; (b)—pure HFBAc; (c)—HFB-iPEth spectrum in d-DMSO isolated from 19F NMR spectrum of the reaction mixture sample; (d)—reaction mixture sample in d-DMSO; (e)—correlation spectroscopy 19F-19F of HFBol; (f)—correlation spectroscopy 19F-19F of HFBAc.
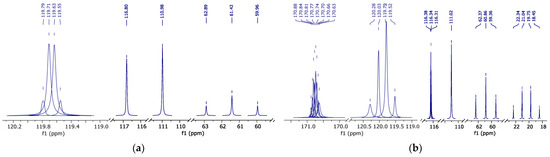
Figure 6.
13C{19F} NMR spectrum with fluorine-19 decoupled (peak curves): (a)—pure HFBol; (b)—pure HFBAc.

Table 3.
Structure of fluorinated compounds and group chemical shifts.
In conclusion, it is worth noting that there is a significant material balance divergence during the RD process for no apparent reason. This can be explained by the formation of propylene during the dehydration of IPol, less likely IPEth. At the same time, the complete dehydration of IPol [18,19] to form water and propylene proceeds under “harsher” conditions compared to those previously investigated. One way or another, propylene formation should be accompanied by the presence of corresponding traces of the component on the 1H NMR spectra of the samples and/or gas emission during the investigation of the chemical constituent of the process in the stirred reactor. Both conditions were not met in an explicit form.
If propylene is formed, it is possible reaction that a reaction could occur in the system between propylene and HFBol, leading to HFB-IPEth formation. The reactions of non-fluorinated olefins with HFBol have not been presented in the literature, but it can be assumed that they proceed similarly to HFBol + hexafluoropropylene interactions. For example, a number of reactions between a type of alcohol with halogen–olefin to form an ether have been discussed in the literature. The reaction of HFBol with hexafluoropropylene is exemplified. This reaction is carried out at 25 °C with 100% conversion, and the content of the target ether in the reaction mixture is 96% [20,21]. Another example is the reaction of HFBol with 3-halagen-1-propene with perfluoroalkyl allyl ether formation [22,23].
4. Conclusions
In the present study, the combination of GC-MS and NMR spectroscopy proved to be an invaluable tool for the successful identification of the components present in the reaction mixture. The approach was used to show and indicate that HFBAc, IPEth, AAc, IPol, water, and HFB-IPEth are formed as the reaction products of IPAc and HFBol. Another important point is that this study provides data on an the new and unstudied compound, HFB-IPEth, for which there was no CAS No. Based on the obtained results, the paper also shows that no bis(1H,1H-heptafluorobutyl) ether traces were identified in the system; the overall reaction and possible interaction among the components of the reaction mixture is described. In addition, original data on the mass spectra and 1H, 19F, 19F-19F COSY and 13C{19F} NMR spectra of the fluorinated compounds are presented. This information is of interest for a wide range of fields of knowledge, where the compounds under study will be addressed in one form or another.
Author Contributions
Conceptualization, A.V.P. and E.V.L.; validation, A.V.P., E.V.L. and T.D.K.; formal analysis, A.V.P., E.V.L. and T.D.K.; investigation, A.V.P., E.I.K., N.A.S. and T.D.K.; resources, A.V.P. and S.Y.K.; writing—original draft preparation, A.V.P., E.V.L. and T.D.K.; writing—review and editing, A.V.P. and E.V.L.; visualization, A.V.P., E.V.L. and T.D.K.; supervision, A.V.P. and E.V.L.; project administration, A.V.P.; funding acquisition, A.V.P. and E.I.K.; GC-MS analysis, A.V.P., E.V.L. and T.D.K.; NMR analysis, A.V.P., E.V.L. and N.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the Russian Science Foundation, project no. 23-79-01164 https://rscf.ru/en/project/23-79-01164/ (accessed on 22 June 2024). The NMR and GC-MS analysis were performed using the equipment of the JRC PMR IGIC RAS.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rogueda, P. Aerosol Formulation Containing a Polar Fluorinated. Compound. Patent No. WO2002003958 A1, 17 January 2002. [Google Scholar]
- Grimm, J.B.; Tkachuk, A.N.; Patel, R.; Hennigan, S.T.; Gutu, A.; Dong, P.; Gandin, V.; Osowski, A.M.; Holland, K.L.; Liu, Z.J.; et al. Optimized red-absorbing dyes for imaging and sensing. J. Am. Chem. Soc. 2023, 145, 23000–23013. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, S.; Kang, H.; You, S.; Kim, C.; Chae, H. Low global warming C4H3F7O isomers for plasma etching of SiO2 and Si3N4 films. ACS Sustain. Chem. Eng. 2022, 10, 10537–10546. [Google Scholar] [CrossRef]
- Nakaue, T.; Nakamura, I. Methods for Producing Iodofluoroalkane and. Fluoroolefin. Patent No. JP2021138632 A, 16 September 2021. [Google Scholar]
- Olson, D.B.; Savu, P.M.; Hebrink, T.J. Copolymers Including Ultraviolet Absorbing Groups and Fluoropolymer Compositions Including. Them. Patent No. US20150353662 A1, 10 December 2015. [Google Scholar]
- Husted, D.R.; Ahlbrecht, A.H. Diacyl Esters of Fluorocarbon. Aldehydrols. Patent US2568501, 18 September 1951. [Google Scholar]
- Hayashi, H.; Yasukochi, S.; Sakamoto, T.; Hatano, M.; Ishihara, K. Insight into the mechanism of the acylation of alcohols with acid anhydrides catalyzed by phosphoric acid derivatives. J. Org. Chem. 2021, 86, 5197–5212. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.H.; Hong, M.C. A process for the Preparation of Fluorinated Methacrylate. Derivatives. Patent No. US20200002265 A1, 2 January 2020. [Google Scholar]
- Qian, H.; Xu, R.; Liu, H. Method for Preparation of Fluorine-Containing Acrylate Useful for Textile Finishing. Agents. Patent No. CN102010334 A, 13 April 2011. [Google Scholar]
- Vandamme, M.; Bouchard, L.; Gilbert, A.; Keita, M.; Paquin, J.-F. Direct esterification of carboxylic acids with perfluorinated alcohols mediated by XtalFluor-E. Org. Lett. 2016, 18, 6468–6471. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, S.; Tsuno, T.; Aburatani, R. Fluorine-Containing Carbonates or Carbamates, Their Manufacture, Electrolytes Containing them and Lithium Salts, and Secondary Lithium Batteries Using the. Electrolytes. Patent No. JP2011032203 A, 17 February 2011. [Google Scholar]
- Mori, K.; Hagiwara, Y.; Nagamori, M.; Isono, Y.; Narizuka, S.; Maeda, K. Fluorine-Containing Compound, Fluorine-Containing Polymer, Resist Composition, Top Coat Composition and Pattern Formation. Method. Patent No. WO2010140483 A1, 9 December 2010. [Google Scholar]
- Qian, X.; Zhao, Z.; Zhu, W.; Li, P.; Tian, Z.; Li, B.; Shi, Y.; Xu, Y. Preparation of Fluoro-Containing Benzo[1,2,3]thiadiazole. Agrochemicals. Patent No. CN102532059 A, 4 July 2012. [Google Scholar]
- Adachi, Y.; Ichikawa, K. Preparation of Triarylsulfonium Sulfonate Salts as Acid Generators, a Resist Composition, and Method for Manufacturing of a Resist. Pattern. Patent No. JP2018090565 A, 14 June 2018. [Google Scholar]
- Han, J.; Haines, C.A.; Piane, J.J.; Filien, L.L.; Nacsa, E.D. An Electrochemical Design for Catalytic Dehydration: Direct, Room-Temperature Esterification without Acid or Base Additives. J. Am. Chem. Soc. 2023, 145, 15680–15687. [Google Scholar] [CrossRef]
- Sugimoto, T. Method for the Preparation of α,β-dihalogenopropionic Acid Fluoroalkyl. Ester. Patent No. JP2023046125 A, 3 April 2023. [Google Scholar]
- Ogura, Y.; Terashima, T.; Sawamoto, M. Synthesis of fluorinated gradient copolymers via in situ transesterification with fluoroalcohols in tandem living radical polymerization. Polym. Chem. 2017, 8, 2299–2308. [Google Scholar] [CrossRef]
- Fukuhara, H.; Matsunaga, F.; Yasuhara, M.; Araki, S.; Isaka, T. Preparation of Propylene by Dehydration of Isopropanol in the Presence of A Pseudo-Boehmite Derived Gamma Alumina. Catalyst. Patent No. US5227563A, 13 July 1993. [Google Scholar]
- Dubois, J.-L.; Postole, G.; Silvester, L.; Auroux, A. Catalytic Dehydration of Isopropanol to Propylene. Catalysts 2022, 12, 1097. [Google Scholar] [CrossRef]
- Lee, H.J.; Yoo, G.S.; Kim, C.S.; Kim, H.G.; Lee, B.G.; Kim, H.S. Method for Preparing Fluorine-Containing Ether Using Ionic. Liquid. Patent No. KR2008110203 A, 18 December 2008. [Google Scholar]
- Lee, H.J.; Lee, S.D.; Yoo, G.S.; Min, B.G.; Lee, B.G.; Kim, H.S. Method for the Preparation of Fluorine-Containing. Ether. Patent No. KR2009131049 A, 28 December 2009. [Google Scholar]
- Lazzari, D.; Cassani, M.C.; Solinas, G.; Pretto, M. Fluoroalkyl allyl ethers: Useful building blocks for the synthesis of environmentally safer fluorinated multiblock molecules. J. Fluor. Chem. 2013, 156, 34–37. [Google Scholar] [CrossRef]
- Lazzari, D.; Cassani, M.C.; Brucka, M.A.; Solinas, G.; Pretto, M. Solvent-free catalytic isomerization of perfluoroalkyl allyl ethers. New J. Chem. 2014, 38, 641–649. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).