Abstract
Thiosemicarbazone (TSC) derivatives and their complexes have emerged as versatile medicinal agents. Now, the focus has shifted to targeted drug delivery and here, the application of nanotechnology is being explored. Nanoparticles (NP) are being explored owing to their tremendous medicinal applications. They are also known to overcome the water insolubility of medicinal agents and have the ability to target specific targets. This article aims to explore the fabrication strategies and applications of functionalized TSCs conjugated with NPs for improved therapeutic potential. The studies were taken from the recent literature and indexed in leading databases. The literature survey reveals the fabrication of TSCs with chitosan-coated superparamagnetic magnetite NPs, which showed significant anti-proliferative activity against several cell lines. Similarly, cobalt oxide nanoparticles conjugated with TSCs have been tested against the hepatic cancer cell line HepG2. Other than anticancer activity, the functionalized nanoparticles have also been employed against drug-resistant pathogens. To improve the oral bioavailability and pharmacological activity, nanoparticle-based block polymers have been proposed to encapsulate the TSC moiety. The in vitro activity of the fabricated NPs has been tested against Leishmania amazonensis. Against microphages, less cytotoxicity was observed. The article may shed light on the structure–bioactivity relationship of novel nanocomposites derived from TSCs and NPs and their specific mechanisms of action.
1. Introduction
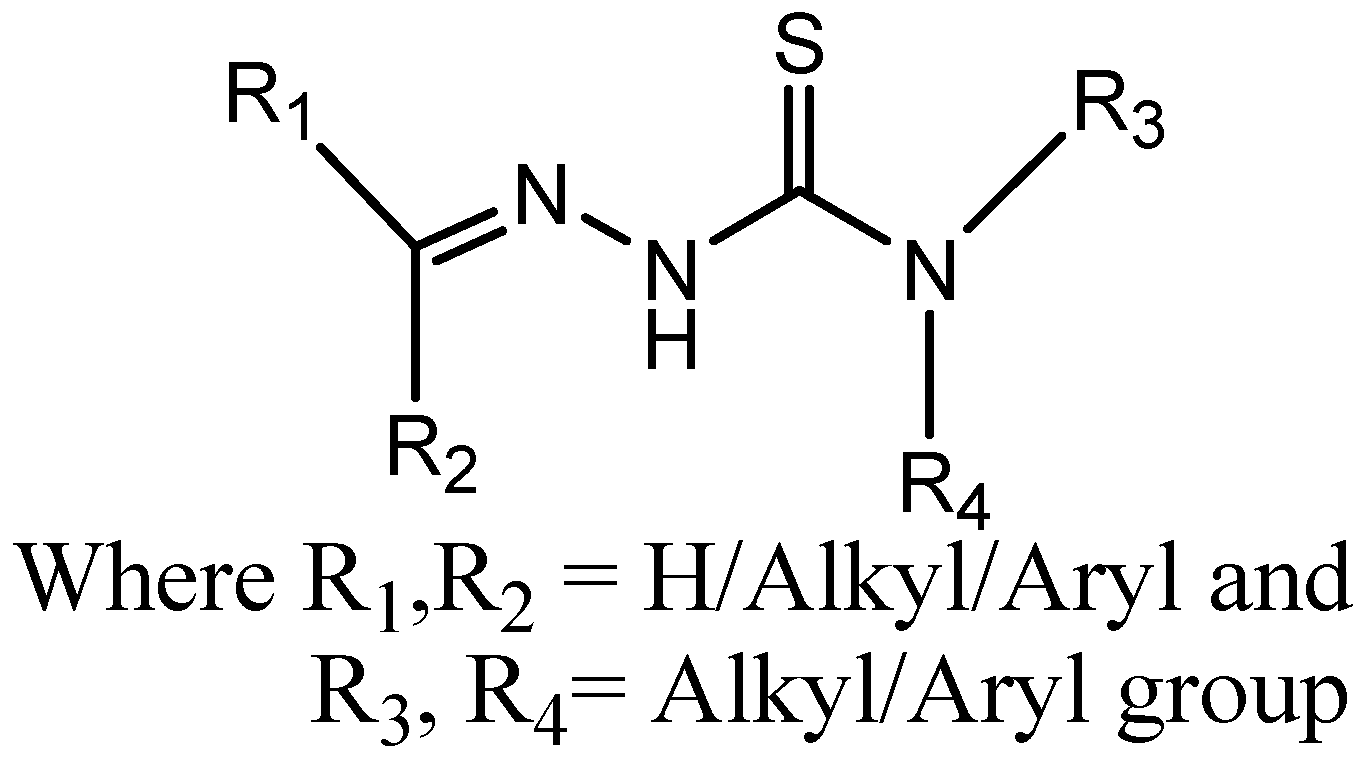
TSCs are Schiff bases that have been well-studied for antibacterial, antifungal, and anticancer properties [1]. TSCs (Figure 1) also act as chelating ligands and form a variety of complexes with metals, which increases their biological activities. TSCs are effective chelating agents readily coordinating with metal ions to generate complexes that can alter the biological activity of free ligands because of the electronic characteristics of the NNS donor system and the diversity of chemical species that the system can produce [2]. TSCs and other iron-chelating medications may have an impact on a number of proteins involved in DNA replication and repair because they need iron as a cofactor. Through the alkylation of the thiol on the top II-DNA complex, TSCs can stabilize the cleavable complex between topoisomerase II and DNA. Furthermore, TSCs can block the iron-containing enzyme ribonucleotide reductase via metal ion chelation, since iron has been demonstrated to be an important molecular target in cancer cell growth and the formation of reactive oxygen species [3]. The antiviral efficacy of various TSCs appears to depend significantly on the inhibition of RNA polymerases, as shown by point mutations in the enzymes that result in drug-resistant virus types [4]. Major problems arise when using large-sized materials for the administration of drugs, like target-specific delivery, low bioavailability, inadequate absorption in the body, poor solubility, and potential negative effects of drugs [5].
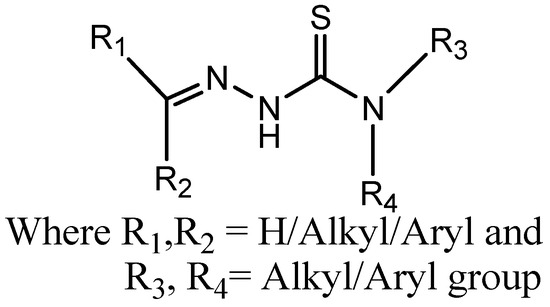
Figure 1.
General structure of thiosemicarbazone.
2. Nanotization of TSCs and Their Conjugation with Functionalized NPs
TSC derivatives and their complexes have been found to possess a wide range of biological activities. The presence of the imine group has been associated with inhibitory action and the cytotoxicity of the complexes is linked to the intercalation between pairs of DNA bases, or the breaking of DNA strands [6]. However, for the effective treatment of cancer, targeted therapy is desirable and required to avoid damage to the normal tissues. Superparamagnetic NPs have been synthesized for biomedical applications as they are guided by an external magnetic field, hence preventing the spontaneous aggregation of the nanoparticles. Supermagnetic iron oxides are promising candidates as they are easily available at the nanoscale, with great potential in medicinal chemistry including drug delivery. The NPs can be coated with different species for further functionalization. Chitosan has potential biological and chemical properties and has reactive groups like OH and NH [7]. Triapine, a TSC, has potent anticancer activity, which was being investigated in different clinical trials. In a study, triapine was encapsulated into polymeric NPs and remote-loaded liposomes for the improvement in drug pahramacokinetics and targeted delivery [8]. The nano-formulations facilitated the release of triapine derivatives with improved remote-loading properties. The encapsulation efficiency increased and resulted in a strongly reduced cytotoxic activity against cancer cells. The drug-induced methemoglobin formation was also studied, which was prevented by the liposomal formulation. The encapsulation of triapine was performed with the polymer matrices of poly(lactic acid) PLA and poly (lactide-co-glycolide)(PLGA) as both are biodegradable and biocompatible. The encapsulation into the liposomal formulations was tested by the remote loading approach, one of the best-studied preparatory methods for liposomes like Doxil [9]. The nanoparticulate drug formulations may be used as an ideal strategy as encapsulated drugs offer prolonged plasma half-life. Generally, doxorubicin is considered an ideal drug for loading as it contains a crucial weak base amine that becomes protonated inside the ammonium sulfate liposome. The loaded molecule should have a log D at pH 7 in the range of −2.5 to 2 and a pKa ≤ 11. Also, Triapinets to tese prerequisites had a log D7.4 ¼ 0.85 ± 0.08 and a pKa ≤ 11 (pK1 ¼ 3.92 and pK2 ¼ 10.78) [10]. Another study also explored the potential of nano-scale PLGA particles for the encapsulation and delivery of the anti-proliferative chelator Dp44mT (Di-2-pyridylketone-4,4-dimethyl-3-thiosemicarbazone) to tumor cells. Nano-scale particles of biodegradable PLGA were used to encapsulate Dp44mT showing efficient encapsulation. The resulting NPs had good colloidal ability and the water solubility was also found to increase and the formulation was found to be very effective against glioma cells. The nanoprecipitation technique was used to prepare Dp44mT-loaded PLGA NPs (Dp44mT-NPs) [11].
A number of studies have reported the activity of TSCs against protozoa like Plasmodium falciparum, Trichomonas vaginalis, etc. [12]. TSCs show inhibitory action cysteine ptease and other enzymes expressed in all stages of T. cruzi, which are crucial for the replication of intracellular parasites. Moreover, the anti-leishmania activity of TSCs has also been reported. The activity of benzaldehyde TSC derivatives of limonene complexed with copper has been tested against Leishmania amazonensis [13]. However, the hydrophobic characteristic of benzaldehyde TSC affects its administration and bioavailability. Nanotechnology has been explored for the diagnosis and prevention of infectious diseases. The NPs can overcome the problem of poor solubility in water and for being target-specific. The NPs have been implemented for drug delivery systems to direct antileishmanial agents to the cells of the reticuloendothelial system [14]. PLGA-PEG encapsulated miltefosine NPs have been developed to target the macrophage of tissues infected with L. donovani [15]. NP-based block polymers to encapsulate the benzaldehyde TSC were synthesized and the morphological parameters were studied by transmission and cryo-transmission electronic microscopy. The entrapment encapsulation efficiency of the BZTS NPs was measured by ultraviolet spectrophotometry. In vitro, activity of the NP suspensions against intracellular amastigotes of Leishmania amazonensis and cytotoxic activity were also evaluated. Spherical NPs were formed with varied sizes depending on the hydrophobic portion of the amphiphilic di-block copolymers [16]. Significant concentration-dependent inhibitory activity against intracellular amastigotes was observed.
Therefore, nanomedicine is an emerging field describing the production and marketing of nano-formulated drugs. Thus, using nanomaterials-based drug delivery systems for targeting specific body sites would be able to solve these challenging issues [17,18]. By producing particles at the nanoscale, the surface area is increased, and hence, the dissolution rate of the drug increases, resulting in higher bioavailability. Recently, researchers have developed different types of nanomaterial like nanocrystals for cancer treatment, metal oxide NPs, iron oxide NPs (IONPs), and mesoporous silica NPs for drug and gene delivery and target-specific delivery to develop improved, efficient, and biocompatible therapeutic carriers [19]. Nanomedicine offers numerous appreciable applications as biochemical, immunotherapeutic, chemotherapeutic agents, etc., and has been used for the treatment of various diseases in recent times [20]. A wide range of sizes and shapes of metal-based NPs have been studied concerning drug delivery and diagnosis. Some metal-based NPs are Ni, Au, Ag, Fe2O3, CuO, ZnO, TiO2, MgO, Al2O3 NPs, etc. Metal-based NPs are proven to provide a large surface area that allows the incorporation of large drug doses [21]. One major issue with the use of NPs is their strong affinity toward adhesion and aggregation. To mitigate this tendency, surface modification of NPs using organic compounds has been used to lower the surface energy of the particles and minimize their tendency to agglomerate [22]. Therefore, to enhance the efficacy and biocompatibility of NPs, the functionalization of these NPs with TSCs has been studied recently, which might be good therapeutic agents for various diseases.
Chitosan (CS) and polyethylene glycol (PEG) polymers have been recently used in the functionalization of metal oxide NPs, which play an important role in drug delivery systems, bioimaging, etc. Chitosan is often used in combination with other polymers or inorganic materials such as magnetic NPs [23].
The functionalization of the metal oxide NPs’ surface can be achieved either by physisorption (non-covalent interaction) or by chemisorption (covalent bonding) with ligands. Generally, ultrasonication methods have been used to functionalize nanoparticles [24]. Since different nanomaterials have distinct characteristics of chemical properties, different functional groups can be bonded to their surfaces for the functionalization process. To include an organic functional group (R-NH2, R-COOH, etc.), homo- or hetero-bifunctional cross-linkers are often required. For instance, amino silanes have been used as cross-linkers to functionalize silica NPs. The amino group present on the surface of the NPs assisted in their conjugation with the ligand. Similarly, noble metals, like Au, Pt, and metal oxide NPs, can be functionalized by using crosslinkers with -SH, -NH2, or -COOH groups that facilitate covalent bonding with ligands [25]. The most commonly used cross-linkers are amino acids like glutamic acid, aspartic acid, cysteine, lysine, etc. The carboxylic acid group of these amino acids or amine and thiol functional groups in cysteine and lysine usually bind to the surface of the NPs [26,27].
3. Design and Fabrication of the Heterojunction of TSCs with Metal Oxide NPs
The following methodology could be used to produce conjugated TSC with metal oxide NPs:
- In the first step, the TSC is synthesized via a condensation reaction with aromatic aldehyde/ketone with thiosemicarbazide in the presence of an acidic medium using an ethanol solvent system, and generally, the reaction takes place at 60 to 70 °C for 3 to 4 h [28];
- In the second step, metal oxide NPs are synthesized by using a bottom-up approach like the sol-gel method, chemical precipitation method, or green synthesis [29];
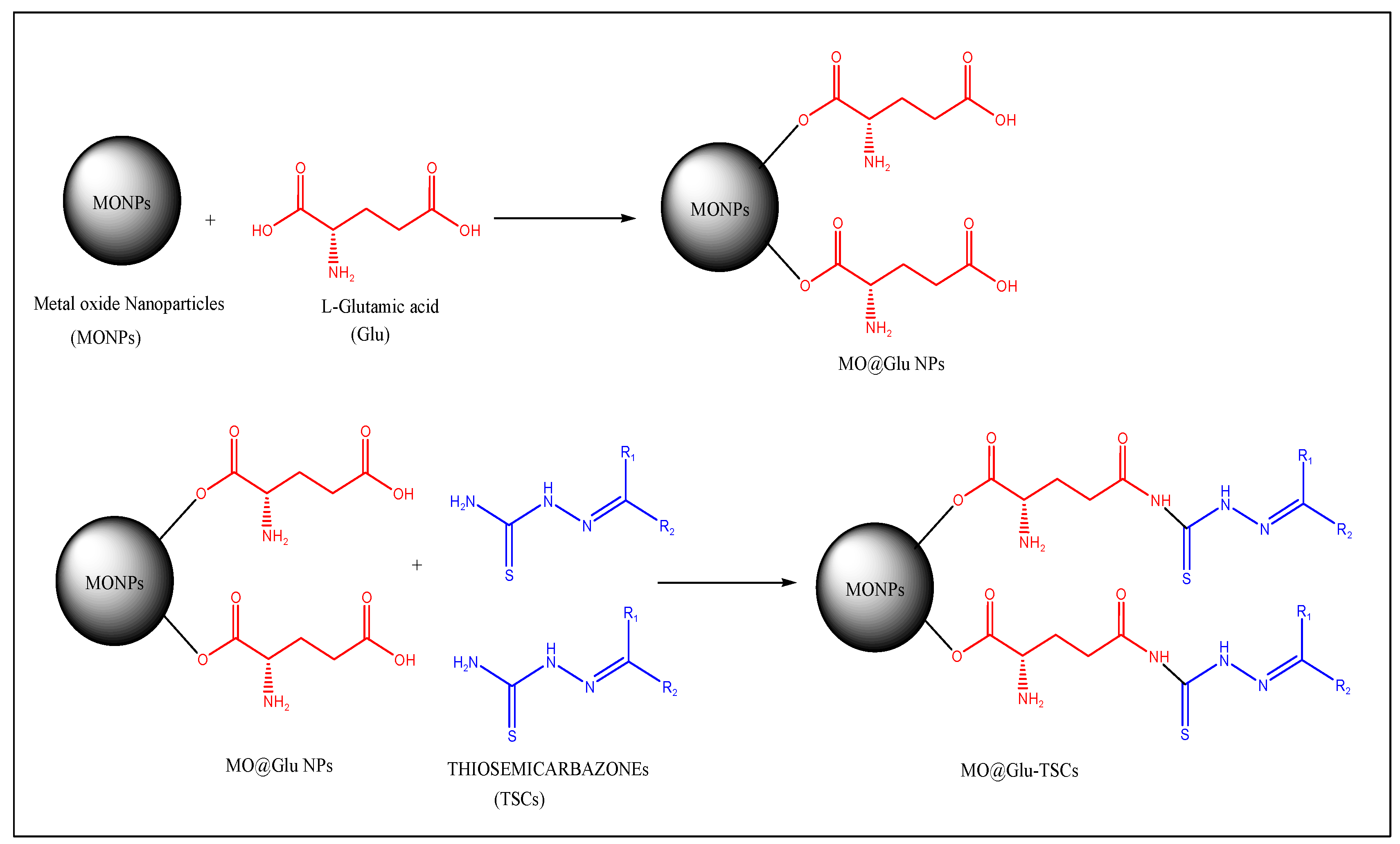
- In the third step, the synthesized NPs are functionalized by using suitable cross-linkers like L-glutamic acid, L-aspartic acid, or 3-mercaptopropionic acid by using the co-condensation method [30];
- In the final step, the functionalized NPs can be conjugated with TSC by the ultrasonication method. Some studies, which have reported the methodology for the conjugation of TSC with NPs, are as follows:
- (a)
- To conjugate TSC to ZnO@Glu NPs, ZnO@Glu (500 mg) and TSC (200 mg) were sonicated in 100 mL ethanol for 30 min and then stirred overnight at 40 °C. The final product (ZnO@Glu–TSC) was separated by centrifugation, washed with water and ethanol, and dried at 60 °C for 6 h;
- (b)
- Co3O4 NPs were functionalized by glutamic acid (Glu) via a co-condensation reaction to produce Co3O4@Glu. Finally, 500 mg of Co3O4@Glu-NP was mixed with 200 mg of TSC in 150 mL of ethanol 96% and incubated in an ultrasonic bath for 40 min at 45 °C. The solution was placed on a heater at 40 °C. After 24 h, the precipitate was separated by centrifugation and washed with ethanol at 70 °C to dry;
- (c)
- For the synthesis of NiO@Glu-TSC, 300 mg of nickel chloride with 152 mg of glutamic acid was dissolved in 150 mL of distilled water and incubated at 50 °C, and then the NaOH (10%) solution was added to increase the pH to about 11. The mixture was incubated at 80 °C for 2 h. After that, the precipitate was separated with a centrifuge and finally, the resulting precipitate was dried at 70 °C. In total, 500 mg of the previous sediment (NiO@Glu) with 200 mg of thiosemicarbazide were dissolved in 150 mL of ethanol and placed in an ultrasonic bath for 45 min. Then, the mixture was placed at 40 °C for 24 h. The precipitate was then separated by centrifugation and finally dried at 70 °C.
The schematic representation is given in Figure 2 for the conjugation of TSCs with metal oxide NPs.
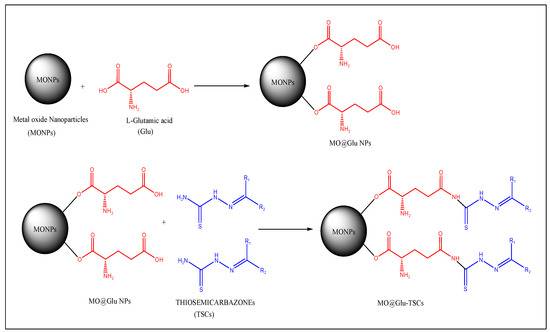
Figure 2.
Schematic representation of the conjugation of TSCs with metal oxide NPs.
4. Some Medicinal Applications of TSCs Conjugated with Functionalized Metal Oxide NPs
A very recent study has reported the cytotoxic effect of CuO NPs conjugated with TSC and functionalized with glutamic acid against the cancer cell line MCF-7 and normal cell lines HEK-293. The synthesis of CuO@glu/TSC was conducted by the co-precipitation method. The dose-dependent antiproliferative activity showed the IC50 value of 133.97 g/mL against the breast cancer cell line whereas against the normal cell line, the IC50 was 230.2 g/mL, which may be attributed to the nanoparticle formulation [31]. In another study, Co3O4 NPs conjugated with TSC (Co3O4-TSC) were tested against the HepG2 cell line. By evaluating the levels of the anti-apoptotic Bcl2 and apoptotic Bax genes using certain primers and the Real-Time PCR technique, the effect of the conjugated NPs on apoptosis was evaluated. Compared to normal cells, cancer cells treated with NP had increased mean expression of the Bax gene. In cancer cells treated with NP, the Bax/Bcl2 expression ratio was greater than 1.0. Because of decreased expression of the anti-apoptotic Bcl2 gene and increased expression of the apoptotic Bax gene, the heterojunction of Co3O4 NPs with TSC exhibited more cytotoxic effects [32]. Nejabatdoust et al. synthesized ZnO NPs, which were functionalized with glutamic acid and then conjugated to thiosemicarbazide (TSC) (ZnO@Glu–TSC) and after that, the conjugated NPs were evaluated against the Staphylococcus aureus multidrug-resistant strain. The ZnO@Glu–TSC was found to be 2–8 times more potent than the standard drug ciprofloxacin [33]. Hosseinkhah et al. synthesized NiO NPs and their functionalization with thiosemicarbazide using glutamic acid as a cross-linker. The characterization of NiO@Glu-TSC was performed using various spectroscopic techniques like UV–Visible Fourier Transform Infrared (FT–IR), X-ray diffraction (XRD), scanning electron microscopy (SEM), and energy dispersive X-ray analysis (EDX). Using the MTT assay method, the cytotoxicity of the NiO@Glu-TSC NPs has been assessed for AGS cells and normal fibroblast cells. The IC50 values of 220 and 390 µg/mL, respectively, for the NiO@Glu-TSC NPs, indicated increased toxicity for AGS cells compared to normal fibroblast cells [34]. In another study, bifunctional dextran-coated iron oxide NPs were produced and preclinically assessed by Gholipour et al. for dual PET/MRI of biotin receptor-positive cancers. The functionalization of the NPs involved oxidizing the dextran coating layer to dialdehyde dextran. They were then coated with the cation chelator thiosemicarbazide and the tumour-targeting compound biotin hydrazide [35]. Habibi et al. synthesized Fe3O4 NPs and conjugated them with the 5-bromosalicylaldehyde thiosemicarbazone using glutamic acid as a crosslinker (Fe3O4@Glu/BTSC) and they investigated their cytotoxic effect against Lung Cancer A549 cells. The results showed that Fe3O4@Glu/BTSC was more effective on A549 cells (IC50 = 166.77 µg/mL) than on BTSC and Fe3O4, but had a lower impact on healthy cells (IC50 = 189.15 µg/mL) [36]. Soares et al. studied chitosan-based doxorubicin delivery systems and they found that doxorubicin release is higher at acidic pH (4.5) than at physiological pH. These chitosan-based nanoparticles were able to encapsulate up to 70% of doxorubicin [37].
Some other iron oxide functionalized NPs have been used as drug carriers as given in Table 1.

Table 1.
Some FeO functionalized NPs used as a nanocarrier for drug delivery.
Habibi et al. studied the apoptotic action of magnetic Fe3O4 NPs functionalized with glutamic acid and 2-pyridinecarboxaldehyde thiosemicarbazone (PTSC) against the human lung epithelial carcinoma A549 cell line and they found that Fe3O4@Glu/PTSC was shown good anti-proliferative properties (IC50 = 135.6 µM/mL) while PTSC alone did not show any activity [43]. Taati et al. studied that the Ag NPs functionalized by glutamine and conjugated with thiosemicarbazide induce apoptosis in colon cancer cell lines. By using an MTT assay, they found that Ag@Gln-TSC NPs were significantly more toxic for colon cancer cells than normal fibroblast cells with IC50 of 88 and 186 µg/mL, respectively [44].
5. Conclusions
The nano-formulated TSCs have the potential to emerge as efficient, economical, safe, and eco-friendly biologically active agents. This would lead to a novel and revolutionary field of developing drugs with high bio-availability and efficient in vivo activity. The idea of the development of nanomedicines is new and rapidly growing. Nanotechnology has several advantages ranging from site-specificity to target-oriented drug delivery. Due to enhanced modes of action, thiosemicarbazones are more effective against a variety of diseases when nano-conjugated with metal oxide NPs.
Author Contributions
Investigation, formal analysis and writing—original draft, E.V.; writing—final draft, S.J.; Conceptualization and Supervision, T.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data was created during the study.
Acknowledgments
The authors are thankful to the R&D cell of Integral University, Lucknow for providing the Manuscript Communication Number (IU/R&D/2024-MCN0002830).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ibrahim, A.A.; Khathi, M.T.; Kareem, M.M. Spectroscopic Elucidation and Biological Assay of Hybrid Salicylaldehyde-Thiosemicarbazone Nickel & Copper Complexes. J. Pharm. Negat. Results 2022, 13, 872–877. [Google Scholar]
- Hashmi, K.; Gupta, S.; Siddique, A.; Khan, T.; Joshi, S. Medicinal applications of vanadium complexes with Schiff bases. J. Trace Elem. Med. Biol. 2023, 79, 127245. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Raza, S.; Lawrence, A.J. Medicinal utility of thiosemicarbazones with special reference to mixed ligand and mixed metal complexes: A Review. Russ. J. Coord. Chem. 2022, 48, 877–895. [Google Scholar] [CrossRef]
- Khan, T.; Azad, I.; Ahmad, R.; Raza, S.; Dixit, S.; Joshi, S.; Khan, A.R. Synthesis, characterization, computational studies and biological activity evaluation of Cu, Fe, Co and Zn complexes with 2-butanone thiosemicarbazone and 1, 10-phenanthroline ligands as anticancer and antibacterial agents. Excli J. 2018, 17, 331. [Google Scholar] [PubMed]
- Tandel, H.; Bhatt, P.; Jain, K.; Shahiwala, A.; Misra, A. In-vitro and in-vivo tools in emerging drug delivery scenario: Challenges and updates. In In-Vitro and In-Vivo Tools in Drug Delivery Research for Optimum Clinical Outcomes; CRC Press: Boca Raton, FL, USA, 2018; pp. 19–42. [Google Scholar]
- Gupta, S.; Singh, N.; Khan, T.; Joshi, S. Thiosemicarbazone derivatives of transition metals as multi-target drugs: A review. Results Chem. 2022, 4, 100459. [Google Scholar] [CrossRef]
- Bhattarai, S.R.; Kc, R.B.; Kim, S.Y.; Sharma, M.; Khil, M.S.; Hwang, P.H.; Chung, G.H.; Kim, H.Y. N-hexanoyl chitosan stabilized magnetic nanoparticles: Implication for cellular labeling and magnetic resonance imaging. J. Nanobiotechnol. 2008, 6, 1. [Google Scholar] [CrossRef]
- Fischer, B.; Kryeziu, K.; Kallus, S.; Heffeter, P.; Berger, W.; Kowol, C.R.; Keppler, B.K. Nanoformulations of anticancer thiosemicarbazones to reduce methemoglobin formation and improve anticancer activity. RSC Adv. 2016, 6, 55848–55859. [Google Scholar] [CrossRef]
- Barenholz, Y.C. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Babak, M.V.; Ahn, D. Modulation of intracellular copper levels as the mechanism of action of anticancer copper complexes: Clinical relevance. Biomedicines 2021, 9, 852. [Google Scholar] [CrossRef]
- Gaur, K.; Vázquez-Salgado, A.M.; Duran-Camacho, G.; Dominguez-Martinez, I.; Benjamín-Rivera, J.A.; Fernández-Vega, L.; Sarabia, L.C.; García, A.C.; Pérez-Deliz, F.; Román, J.A.M.; et al. Iron and copper intracellular chelation as an anticancer drug strategy. Inorganics 2018, 6, 126. [Google Scholar] [CrossRef]
- Walcourt, A.; Loyevsky, M.; Lovejoy, D.B.; Gordeuk, V.R.; Richardson, D.R. Novel aroylhydrazone and thiosemicarbazone iron chelators with anti-malarial activity against chloroquine-resistant and-sensitive parasites. Int. J. Biochem. Cell Biol. 2004, 36, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Britta, E.A.; Barbosa Silva, A.P.; Ueda-Nakamura, T.; Dias-Filho, B.P.; Silva, C.C.; Sernaglia, R.L.; Nakamura, C.V. Benzaldehyde thiosemicarbazone derived from limonene complexed with copper induced mitochondrial dysfunction in Leishmania amazonensis. PLoS ONE 2012, 7, e41440. [Google Scholar] [CrossRef] [PubMed]
- Want, M.Y.; Islamuddin, M.; Chouhan, G.; Dasgupta, A.K.; Chattopadhyay, A.P.; Afrin, F. A new approach for the delivery of artemisinin: Formulation, characterization, and ex-vivo antileishmanial studies. J. Colloid Interface Sci. 2014, 432, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sahoo, G.C.; Pandey, K.; Das, V.N.R.; Topno, R.K.; Ansari, M.Y.; Das, P. Development of PLGA–PEG encapsulated miltefosine based drug delivery system against visceral leishmaniasis. Mater. Sci. Eng. C 2016, 59, 748–753. [Google Scholar] [CrossRef]
- Britta, E.A.; da Silva, C.C.; Rubira, A.F.; Nakamura, C.V.; Borsali, R. Generating nanoparticles containing a new 4-nitrobenzaldehyde thiosemicarbazone compound with antileishmanial activity. Mater. Sci. Eng. C 2016, 69, 1159–1166. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Shin, H.S. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Bhalani, D.V.; Nutan, B.; Kumar, A.; Singh Chandel, A.K. Bioavailability enhancement techniques for poorly aqueous soluble drugs and therapeutics. Biomedicines 2022, 10, 2055. [Google Scholar] [CrossRef]
- Elahi, N.; Rizwan, M. Progress and prospects of magnetic iron oxide nanoparticles in biomedical applications: A review. Artif. Organs 2021, 45, 1272–1299. [Google Scholar] [CrossRef]
- Rahman, M.A.; Saikat, A.S.M.; Rahman, M.S.; Islam, M.; Parvez, M.A.K.; Kim, B. Recent update and drug target in molecular and pharmacological insights into autophagy modulation in cancer treatment and future progress. Cells 2023, 12, 458. [Google Scholar] [CrossRef]
- Sodhi, R.K.; Paul, S. Metal complexes in medicine an overview and update from drug design perspective. Cancer Ther. Oncol. Int. J. 2019, 14, 25–32. [Google Scholar] [CrossRef]
- Mallakpour, S.; Madani, M. A review of current coupling agents for modification of metal oxide nanoparticles. Prog. Org. Coat. 2015, 86, 194–207. [Google Scholar] [CrossRef]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro-and nanoparticles in drug delivery. J. Control. Release 2004, 100, 5–28. [Google Scholar] [CrossRef]
- Afzal, A.; Nawfal, I.; Mahbubul, I.M.; Kumbar, S.S. An overview on the effect of ultrasonication duration on different properties of nanofluids. J. Therm. Anal. Calorim. 2019, 135, 393–418. [Google Scholar] [CrossRef]
- Azadmanjiri, J.; Kumar, P.; Srivastava, V.K.; Sofer, Z. Surface functionalization of 2D transition metal oxides and dichalcogenides via covalent and non-covalent bonding for sustainable energy and biomedical applications. ACS Appl. Nano Mater. 2020, 3, 3116–3143. [Google Scholar] [CrossRef]
- Daima, H.K.; Navya, P.N.; Lichtfouse, E. (Eds.) Nanozymes for Environmental Engineering; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Casettari, L.; Vllasaliu, D.; Lam, J.K.; Soliman, M.; Illum, L. Biomedical applications of amino acid-modified chitosans: A review. Biomaterials 2012, 33, 7565–7583. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, H.P.; Hadi, J.S.; Alsalim, T.A.; Ghali, T.S.; Bolandnazar, Z. A novel series of thiosemicarbazone drugs: From synthesis to structure. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 137, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Soltys, L.; Olkhovyy, O.; Tatarchuk, T.; Naushad, M. Green synthesis of metal and metal oxide nanoparticles: Principles of green chemistry and raw materials. Magnetochemistry 2021, 7, 145. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, B.; Xiao, Y.; Tian, X.; Wang, Y. Fluorescent quantum dots and its composites for highly sensitive detection of heavy metal ions and pesticide residues: A review. Chemosensors 2023, 11, 405. [Google Scholar] [CrossRef]
- Shafiei, I.; Tavassoli, S.P.; Rahmatollahi, H.R.; Ghasemian, R.; Salehzadeh, A. A novel copper oxide nanoparticle conjugated by thiosemicarbazone promote apoptosis in human breast cancer Cell line. J. Clust. Sci. 2022, 33, 2697–2706. [Google Scholar] [CrossRef]
- Jarestan, M.; Khalatbari, K.; Pouraei, A.; Sadat Shandiz, S.A.; Beigi, S.; Hedayati, M.; Majlesi, A.; Akbari, F.; Salehzadeh, A. Preparation, characterization, and anticancer efficacy of novel cobalt oxide nanoparticles conjugated with thiosemicarbazide. 3 Biotech 2020, 10, 230. [Google Scholar] [CrossRef]
- Nejabatdoust, A.; Zamani, H.; Salehzadeh, A. Functionalization of ZnO nanoparticles by glutamic acid and conjugation with thiosemicarbazide alters expression of efflux pump genes in multiple drug-resistant Staphylococcus aureus strains. Microb. Drug Resist. 2019, 25, 966–974. [Google Scholar] [CrossRef]
- Hosseinkhah, M.; Ghasemian, R.; Shokrollahi, F.; Mojdehi, S.R.; Noveiri, M.J.S.; Hedayati, M.; Rezaei, M.; Salehzadeh, A. Cytotoxic potential of nickel oxide nanoparticles functionalized with glutamic acid and conjugated with thiosemicarbazide (NiO@ Glu/TSC) against human gastric cancer cells. J. Clust. Sci. 2022, 33, 2045–2053. [Google Scholar] [CrossRef]
- Gholipour, N.; Akhlaghi, M.; Kheirabadi, A.M.; Geramifar, P.; Beiki, D. Development of Ga-68 labeled, biotinylated thiosemicarbazone dextran-coated iron oxide nanoparticles as multimodal PET/MRI probe. Int. J. Biol. Macromol. 2020, 148, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Habibi, A.; Bakhshi, N.; Amirmozafari, N. Iron oxide nanoparticles conjugated to thiosemicarbazone reduce the survival of cancer cells by increasing the gene expression of microRNA let-7c in lung cancer A549 cells. Arch. Iran. Med. 2022, 25, 807. [Google Scholar] [CrossRef]
- Soares, P.I.; Sousa, A.I.; Silva, J.C.; Ferreira, I.M.; Novo, C.M.; Borges, J.P. Chitosan-based nanoparticles as drug delivery systems for doxorubicin: Optimization and modelling. Carbohydr. Polym. 2016, 147, 304–312. [Google Scholar] [CrossRef]
- Bajpai, A.K.; Gupta, R. Magnetically mediated release of ciprofloxacin from polyvinyl alcohol based superparamagnetic nanocomposites. J. Mater. Sci. Mater. Med. 2011, 22, 357–369. [Google Scholar] [CrossRef]
- Gaihre, B.; Khil, M.S.; Lee, D.R.; Kim, H.Y. Gelatin-coated magnetic iron oxide nanoparticles as carrier system: Drug loading and in vitro drug release study. Int. J. Pharm. 2009, 365, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Arias, J.L.; López-Viota, M.; Delgado, Á.V.; Ruiz, M.A. Iron/ethylcellulose (core/shell) nanoplatform loaded with 5-fluorouracil for cancer targeting. Colloids Surf. B Biointerfaces 2010, 77, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Tong, Q.; Li, H.; Li, W.; Chen, H.; Shu, X.; Lu, X.; Wang, G. In vitro and in vivo anti-tumor effects of gemcitabine loaded with a new drug delivery system. J. Nanosci. Nanotechnol. 2011, 11, 3651–3658. [Google Scholar] [CrossRef]
- Losic, D.; Yu, Y.; Aw, M.S.; Simovic, S.; Thierry, B.; Addai-Mensah, J. Surface functionalisation of diatoms with dopamine modified iron-oxide nanoparticles: Toward magnetically guided drug microcarriers with biologically derived morphologies. Chem. Commun. 2010, 46, 6323–6325. [Google Scholar] [CrossRef]
- Habibi, A.; Sadat Shandiz, S.A.; Salehzadeh, A.; Moradi-Shoeili, Z. Novel pyridinecarboxaldehyde thiosemicarbazone conjugated magnetite nanoparticulates (MNPs) promote apoptosis in human lung cancer A549 cells. JBIC J. Biol. Inorg. Chem. 2020, 25, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Taati, H.; Sangani, H.; Davoudi, A.; Safabakhsh Kouchesfahani, S.; Hedayati, M.; Tarashandeh Hemmati, S.; Salehzadeh, A. Silver nanoparticle functionalized by glutamine and conjugated with thiosemicarbazide induces apoptosis in colon cancer cell line. Sci. Rep. 2024, 14, 3809. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).