Abstract
In the chemical and process industries, different techniques are implemented to enable the separation of azeotropic mixtures. These separation methods are broadly classified as azeotropic distillation procedures, which employ the use of entrainers, and membrane-based processes, which mainly use semi-permeable membrane materials. This paper seeks to examine the current trends employed in the separation procedures for azeotropic mixtures in industry, particularly the techniques and methods applied in the actual processes. Additionally, this paper also outlines the common issues encountered in the current setups for the separation of azeotropic mixtures. Several studies show that in comparison to conventional separation techniques, the application of alternative distillation methods and advanced membrane-based techniques for the separation of azeotropic mixtures results in better separation efficiency and reduced energy consumption while also maintaining the cost-effectiveness of the overall process. In addition to this, advancements in the available techniques for separation would also improve the viability and ensure the long-term sustainability of the proposed developments, addressing the current gaps in knowledge while ensuring that existing challenges in the procedures, like membrane fouling and limited scalability, are properly addressed. Furthermore, this paper also highlights the research outlook of the processes involved in the separation of azeotropic mixtures.
1. Introduction
Azeotropic mixture separation remains a significant challenge in the process industries due to the nature of these mixtures, where the vapor and liquid compositions are identical at a specific temperature and pressure, rendering conventional distillation ineffective. An example of a diagram of an azeotropic mixture is shown in Figure 1. As industries demand higher-purity and sustainable solutions, researchers are turning to alternative separation methods. Two approaches that have shown success in overcoming the limitations of conventional distillation are azeotropic distillation and membrane-based separation technologies. In heterogeneous azeotropic distillation methods, entrainers are used to carry out the separation of non-binary mixtures, whereas membrane-based separation is often preferred for its environmental benefits and energy efficiency, offering a viable alternative to conventional methods.
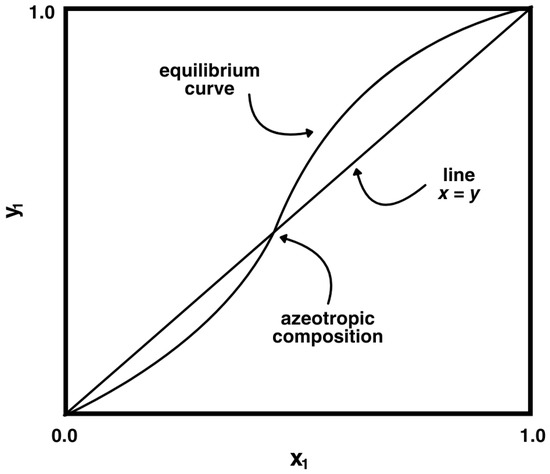
Figure 1.
Diagram of an azeotropic mixture.
This review focuses on the principles and applications of azeotropic distillation and membrane-based separation methods, while also discussing the limitations of current industrial techniques. Additionally, this paper also provided an outlook on future research directions and development opportunities in azeotropic mixture separation.
2. Azeotropic Distillation
Azeotrope mixtures are challenging to separate via conventional distillation due to their constant boiling point and identical vapor–liquid compositions. One method deemed fit for such a condition is the application of azeotropic distillation, in which an entrainer is used, which acts as a solvent or mass separating agent (MSA), to effectively separate non-ideal binary mixtures into pure components. This method is widely employed in reactions involving the production of water as a byproduct, as the removal of water helps shift the equilibrium of the reaction towards the side favoring product formation. The process flow diagram of the azeotropic distillation can be seen in Figure 2.
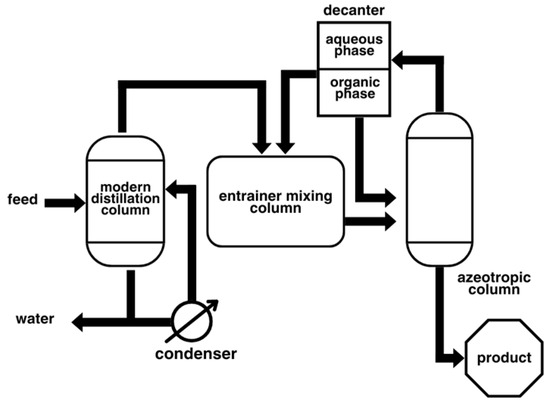
Figure 2.
Schematic diagram of azeotropic distillation.
According to a study by Zhang et al. [1], key considerations in azeotropic distillation include selecting an appropriate entrainer, which requires analyzing vapor–liquid equilibrium and applying thermodynamic tools such as the conductor-like screening model for real solvents (COSMO-RS) and density functional theory (DFT) [2], as well as the recovery of the entrainer from the products. In addition, both the process and methods for entrainer recovery methods must also be considered, as the entrainer often forms an azeotrope with one of the components being separated. Following this, optimal efficiency can be achieved by maintaining a high concentration of entrainers across all plates in the column. The placement of the entrainer in the column must also be considered as this can significantly impact the concentration gradient across the tower. If the entrainer has the same volatility as the feed, it should be added with the feed; otherwise, for lower volatility, it is added near the top of the column [3]. More-volatile entrainers should also be introduced below the feed point.
Further research into azeotropic distillation may be necessary, particularly in solvent purification processes, as this method has been proven highly effective at removing impurities and improving product purity. For instance, it plays a key role in the production of chemicals like ethanol, where separating the desired product is essential. Also, compared to conventional distillation, azeotropic distillation provides better results with increased energy efficiency and reduced consumption. Studies also suggest that optimal entrainers can be used to reduce energy consumption and further optimize the overall process [4].
3. Membrane-Based Separation Techniques
Membrane-based separation techniques are another option for separating azeotropic mixtures, alongside azeotropic distillation. Unlike azeotropic distillation, which uses separating agents, membrane methods rely on a semi-permeable membrane to separate components based on differences in physical properties like size and volatility [5]. These membranes can be organic, polymeric, or inorganic, depending on the specific requirements of the process [5].
While both techniques offer similar benefits, membrane-based methods are generally favored for their sustainability. This is because these require less energy during the process and involve simpler setups. Moreover, research efforts have also led to the development of different approaches, such as membrane distillation and pervaporation, which both do not require additional substances or complex setups when employed [6], and hybrid processes, which combine different membrane-based techniques with distillation methods [7], to address sustainability issues.
In the study of Osman et al. [7], membrane-based methods are preferred over conventional distillation for their energy efficiency, as they operate under reduced conditions and use less energy. However, while these methods may demonstrably be effective, issues like susceptibility to fouling, which can decrease efficiency over time, and scalability issues remain in the industry. Emerging techniques for separation have also demonstrated potential for not only enhancing the separation performance of azeotropic mixtures but also in minimizing the energy consumption of the overall process, contributing to more sustainable processes that meet global standards and regulations [5].
3.1. Membrane Distillation
Membrane distillation (MD) is a complex separation process that is based on the volatility differences between substances to carry out separation. This process leverages the simple mechanism of membranes that perform as a filter for separation of binary mixtures. As observed in Figure 3, membranes allow certain molecules to pass through while preventing others from entering. Meanwhile, MD effectively handles azeotropic mixtures by using a temperature gradient across a hydrophobic membrane to evaporate the more volatile components [4]. Additionally, MD systems use low-grade thermal energy from renewable sources to separate mixtures.
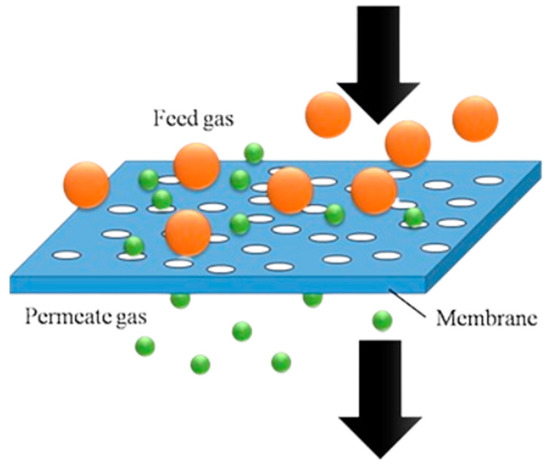
Figure 3.
Working principle of membrane distillation.
However, as with other methods, MD systems likewise encounter issues like the selectivity of the membrane, making membranes prone to membrane wetting from low-surface-tension liquids, and scalability issues, both resulting in reduced separation efficiency. Some studies suggest that advancements in the module design of MD systems—by making the membrane materials capable of handling larger volumes of feed input—may help address this issue and make it a viable method for large-scale procedures [8].
3.2. Pervaporation
As discussed in a research study of Osman et al. [7], pervaporation technology separates components based on differences in solubility and diffusivity using a nonporous membrane. It operates under mild conditions and does not require additional chemicals, which helps maintain stream purity. The general working principle of a pervaporation system is presented in Figure 4.
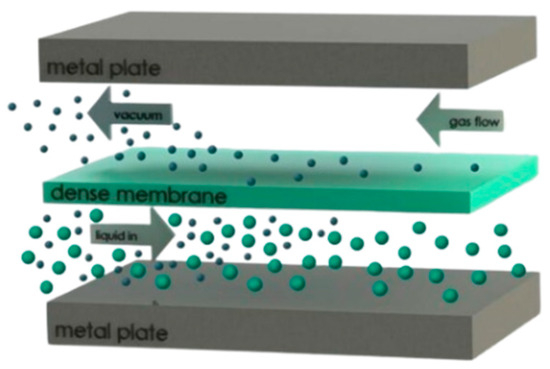
Figure 4.
Working principle of PV systems.
However, PV systems may also be inclined to experience issues like membrane damage due to a loss of membrane integrity from solvent exposure, as well as membrane swelling behavior, which can likely affect the long-term efficiency of the technique without proper interventions. Research by Banjerdteerakul, Peng, and Li suggests that covalent organic frameworks (COFs) could enhance membrane stability and process efficiency due to their high porosity and stability [9]. However, it was discussed in the same study that the applications of COFs in PV systems for organic azeotropic mixtures lack sufficient information from extensive research and require further investigation to be used in conducting unit operations.
3.3. Hybrid Separation
Hybrid separation techniques, also considered membrane-based methods, combine two or more methods for separation to maximize the capacity of each separation method. This approach is often adopted to address the common issues encountered in single-method separation procedures, like limited application, poor separation efficiency, and suboptimal energy use.
3.3.1. Hybrid Separation Scheme
According to Vooradi et al. [10], integrating distillation with membrane separation can improve efficiency by combining the high selectivity of membranes with distillation units. Through this hybrid system, products of higher purity are readily achievable even at lower energy conditions, although the presence of membranes introduces higher pressures and complex setups due to the mass transport resistance, which can affect membrane lifetime and stability. Combined with the potential for fouling and the need for the pre-treatment of feeds, these factors can lead to increased maintenance and capital costs and may deter some industries from adopting this technology.
3.3.2. Distillation with Pervaporation
Khalid et al. [11] discussed another hybrid technique that combines distillation with pervaporation and is particularly effective in bioethanol production. Pervaporation can concentrate the azeotrope upstream of distillation, significantly reducing energy demands and costs associated with the subsequent distillation stage. The process flow diagram of a distillation-pervaporation system is shown in Figure 5.
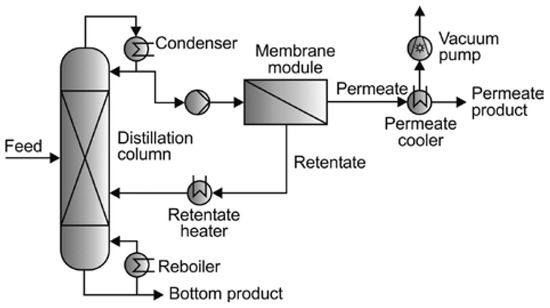
Figure 5.
Schematic diagram of a distillation–pervaporation system.
This hybrid approach has also been established for its potential to reduce solvent costs while also enhancing solvent recovery efficiency. However, similar to other methods, the scalability and economic feasibility of distillation–pervaporation systems in industrial settings have not been studied extensively and may require further research efforts.
3.3.3. Pervaporation with Vapor Permeation
As with other separation techniques, the integration of pervaporation with vapor permeation in a hybrid setup provides similar results in terms of efficiency. This works by removing a portion of water from azeotropic mixtures, resulting in a reduction in the load on the subsequent vapor permeation steps to achieve a higher degree of purification.
The two-stage process allows for more precise separation, potentially reducing operational costs and energy consumption compared to conventional methods or other hybrids. This system is particularly useful for temperature-sensitive mixtures or those needing precise compositional control. A sample of the schematic diagram of a pervaporation-vapor permeation system is shown in Figure 6.
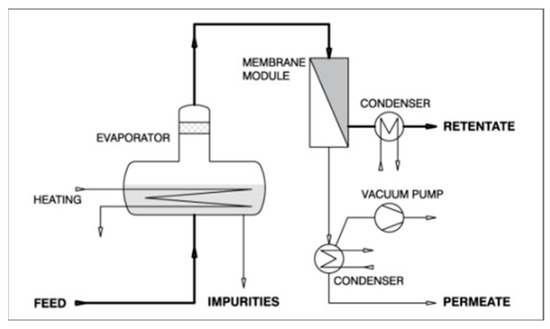
Figure 6.
Schematic diagram of a pervaporation-vapor permeation system.
Table 1 presents an overview of the general methods used in separating azeotropic mixtures. The specific separation techniques for both azeotropic distillation and membrane-based methods were detailed in the table, along with the mechanisms underlying each approach.

Table 1.
Summary of the general methods used in separating azeotropic mixtures.
4. Limitations in Azeotropic Mixture Separation Techniques
While separation methods like azeotropic distillation and membrane-based methods have demonstrably addressed challenges with azeotropic mixtures, it is also essential to understand and recognize the limitations of these methods. Several factors contribute to these limitations and must be studied intensively for optimizing the management of azeotropic mixtures. Some of these limitations in separation are outlined below.
4.1. Entrainer Selection and Process Optimization
Azeotropic distillation, unlike other methods, relies on adding an entrainer or MSA to facilitate separation. Choosing the right entrainer is particularly difficult, as the efficiency of the process may vary based on the specific characteristics and nature of the mixture. According to Gomey et al. [12], the ideal entrainer should do the following:
- Form an azeotrope with one component with a different boiling point;
- Be easily separated from the desired product after distillation;
- Be chemically inert and non-corrosive to avoid equipment damage;
- Be cost-effective.
Additionally, recovering the entrainer for reuse is a complex and energy-intensive process. To optimize azeotropic distillation, improving the concentration and operating conditions of the entrainer—such as temperature, pressure, and feed flow rates—can enhance separation efficiency while reducing energy consumption.
4.2. Energy Consumption
Some separation techniques require extra energy input to facilitate the process. For instance, azeotropic distillation involves heating and maintaining specific temperatures in the distillation column, which can increase costs. Also, mixtures with higher boiling points may need additional distillation stages, further raising expenses. To address this, studies recommend optimizing key process parameters and exploring alternative energy-efficient methods, such as membrane-based separations [13].
4.3. Scale-Up Challenges
Scalability issues for industrial applications are also among the issues commonly encountered, particularly affecting the heat and mass transfer, column design, process stability maintenance, and flow management. As the membrane area and feed flow rates increase, these factors become increasingly important, which highlights the need for appropriate methods when scaling up the methods for large-scale productions to ensure that the overall process is effective and reliable in industrial applications.
4.4. Membrane Fouling
In chemical process industries, fouling is among the common concerns. Since the components present in azeotropic mixtures can induce fouling or degrade the membrane, which may diminish the efficiency of the process over a period of time, the accumulation of impurities may require frequent cleaning—or worse, replacement. To avoid this issue, proactive measures are needed to maintain the long-term effectiveness of membrane-based separation techniques [13].
4.5. Economic Considerations
One of the major concerns regarding the industry-wide application of these techniques is the economic viability, due to the associated costs of the equipment, energy, and the maintenance procedures. For instance, membrane-based systems may require higher initial capital investments compared to conventional methods. To assess the financial risks of large-scale implementation, it is important to evaluate operating costs such as membrane replacement and energy consumption rates as this helps in determining the overall economic feasibility of these technologies [13].
4.6. Environmental Impact
Environmental considerations are important alongside economic factors for industries implementing separation techniques. Certain substances used in these processes can create waste management issues. For example, entrainers, which can include volatile organic compounds (VOCs), may contribute to air pollution if not properly recovered or disposed of. In the case of membrane systems, some membranes may require the use of solvents that are also harmful to the environment. Thus, addressing these environmental impacts is essential for sustainable industry practices.
5. Future Research
As discussed in the previous sections, current techniques for separating azeotropic mixtures, while effective, face sustainability issues due to their high energy requirements. To address this, research efforts should focus on reducing energy consumption through viable alternative methods. Additionally, working under optimal operating conditions may also enhance the efficiency of the process, while also ensuring that these processes maintain practical applicability when applied in industrial-scale settings, and economic feasibility, including but not limited to capital costs and operating expenses, ensuring the long-term viability of these methods. Additionally, using materials with improved stability can help reduce energy consumption and minimize sustainability issues [14].
Extensive research and development should aim to optimize energy-intensive processes for azeotropic mixtures to address current technological gaps [15]. To design these methods, principles in material science may be used to enhance the understanding of thermodynamic properties, fluid dynamics, and phase equilibria, which are crucial for predicting and controlling azeotropic systems. This may also help us to understand the application of unit operations and other dynamics in this type of separation procedure.
Lastly, a reasonable amount of research was solely focused on other membrane technologies. By contrast, however, less attention has been directed to other innovative non-membrane techniques. For example, Kong et al. [16] explored a configuration of reactive–extractive distillation (RED), or the integration of reactive distillation (RD) and extractive distillation (ED), which was deemed fit for azeotropic mixture separation. RED separation systems require less energy input to operate as they only rely on the produced reaction heat for the subsequent separation processes. To establish RED in the industry, sustainability assessments may be carried out to determine the knowledge gaps and avoid operability issues. Also, process evaluations may be employed through process intensification (PI) to further improve and establish the energy-efficient nature of RED systems, while maintaining the overall efficiency of the separation performance.
Author Contributions
Conceptualization. R.V.C.R.; writing—original draft preparation, C.C., K.O.F., S.R.A.G., M.A.M., T.M.G.M.; writing—review and editing, R.V.C.R.; supervision, R.V.C.R.; project administration, R.V.C.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhang, X.; Li, X.; Li, G.; Zhu, Z.; Wang, Y.; Xu, D. Determination of an optimum entrainer for extractive distillation based on an isovolatility curve at different pressures. Sep. Purif. Technol. 2018, 201, 79–95. [Google Scholar] [CrossRef]
- Ramalingam, A.; Gurunathan, R.; Nagarajan, P. Investigation of potential azeotrope breakers using DFT and COSMO approach. ACS Omega 2020, 5, 16885–16900. [Google Scholar] [CrossRef] [PubMed]
- Nuchteera, I.; Thirasak, P.; Chavagorn, M.; Thanyalak, C.; Kitipat, S. Cyclopentane Purification from Multicomponent Azeotropic Mixtures. In 30th European Symposium on Computer Aided Chemical Engineering, 1st ed.; Pierucci, S., Manenti, F., Bozzano, G., Manca, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 48, pp. 277–282. [Google Scholar] [CrossRef]
- Li, C.; Zhong, L.; Wang, L.; Sun, D.; Li, Y.; Cui, P.; Shan, B.; Zhu, Z.; Li, X. Process design and multi-objective optimization of efficient heat utilization distillation based on the influence of pressure and entrainer flow on separation performance. J. Clean. Prod. 2022, 379, 134848. [Google Scholar] [CrossRef]
- Fan, Z.; Tang, K.; Xu, Z.; Zhang, L.; Zhang, G. Highly efficient membrane-based techniques in separation. IOP Conf. Ser. Earth Environ. Sci. 2018, 208, 012050. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, L. Research progress on azeotropic distillation technology. Adv. Chem. Eng. Sci. 2019, 9, 333–342. [Google Scholar] [CrossRef]
- Osman, A.; Chen, Z.; Elgarahy, A.; Farghali, M.; Mohamed, I.; Priya, A.; Hawash, H.; Yap, P. Membrane technology for energy saving: Principles, techniques, applications, challenges, and prospects. Adv. Energy Sustain. Res. 2024, 5, 2400011. [Google Scholar] [CrossRef]
- Ding, X.; Wang, X.; Du, P.; Tian, Z.; Chen, J. Prediction of new distillation-membrane separation integrated process with potential in industrial application. Processes 2021, 9, 318. [Google Scholar] [CrossRef]
- Banjerdteerakul, K.; Peng, H.; Li, K. Covalent organic frameworks based membranes for separation of azeotropic solvent mixtures by pervaporation. J. Membr. Sci. 2023, 678, 121679. [Google Scholar] [CrossRef]
- Vooradi, R.; Patnaikuni, V.S.; Tula, A.K.; Anne Sarath, B.; Eden, M.R.; Gani, R. Hybrid separation scheme for azeotropic mixtures: Sustainable design methodology. Chem. Eng. Trans. 2018, 69, 637–649. [Google Scholar] [CrossRef]
- Khalid, A.; Aslam, M.; Oyyum, M.A.; Faisal, A.; Khan, A.L.; Ahmed, F.; Lee, M.; Kim, J.; Jang, N.; Chang, I.S.; et al. Membrane separation processes for dehydration of bioethanol from fermentation broths: Recent developments, challenges, and prospects. Renew. Sustain. Energy Rev. 2019, 105, 427–443. [Google Scholar] [CrossRef]
- Gomey, A.; Tripathi, M.; Haider, M.; Kumar, R. Comparative analysis of isopropyl alcohol dehydration using ionic liquids and deep eutectic solvent. J. Ion. Liq. 2023, 3, 100069. [Google Scholar] [CrossRef]
- Low, Z.N.; Wang, H. Challenges in membrane-based liquid phase separations. Green Chem. Eng. 2021, 2, 3–13. [Google Scholar] [CrossRef]
- Waltermann, T.; Grueters, T.; Muenchrath, D.; Skiborowski, M. Efficient optimization-based design of energy-integrated azeotropic distillation processes. Comp. Chem. Eng. 2020, 133, 106676. [Google Scholar] [CrossRef]
- Chen, J.; Ye, Q.; Liu, T.; Xia, H.; Feng, S. Improving the performance of heterogeneous azeotropic distillation via self-heat recuperation technology. Chem. Eng. Res. Des. 2019, 141, 516–528. [Google Scholar] [CrossRef]
- Kong, Z.Y.; Yang, A.; Tsai, C.; Adi, V.; Saptoro, A.; Sunarso, J. Design and optimization of hybrid-reactive-extractive distillation for ternary azeotropic separation: A case considering the effect of side reactions. Ind. Eng. Chem. Res. 2023, 62, 10601–10610. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).