Abstract
Caffeine, as 1,3,7-trimethyl-1H-purine-2,6-dione, represents a naturally occurring alkaloid within the methyl xanthine family. Exhibiting bitterness and an odorless state in its pure form, caffeine manifests as needle-like crystals. Found abundantly in tea, coffee beans, kola nuts, and cocoa beans, this compound stimulates the central nervous system, respiration, and cardiac activity. Ubiquitously present in everyday products such as soft drinks, tea, coffee, chocolates, pharmaceutical drugs, and skincare items, caffeine plays a pivotal role. This study focuses on the extraction of caffeine from various tea types, including used tea, utilizing dichloromethane as an organic solvent. Investigating the impact of temperature and residence time on the extraction efficiency, we observed a direct correlation between these factors and efficiency. Notably, the residence time exhibited a significant effect up to a certain threshold, beyond which no substantial difference in extraction efficiency was observed. The optimal conditions for caffeine extraction were identified at 100 °C with a residence time of 30 min. The findings revealed the extraction of 0.089 g of caffeine from black tea, 0.06 g from used black tea, 0.08 g from green tea, and 0.047 g from used green tea.
1. Introduction
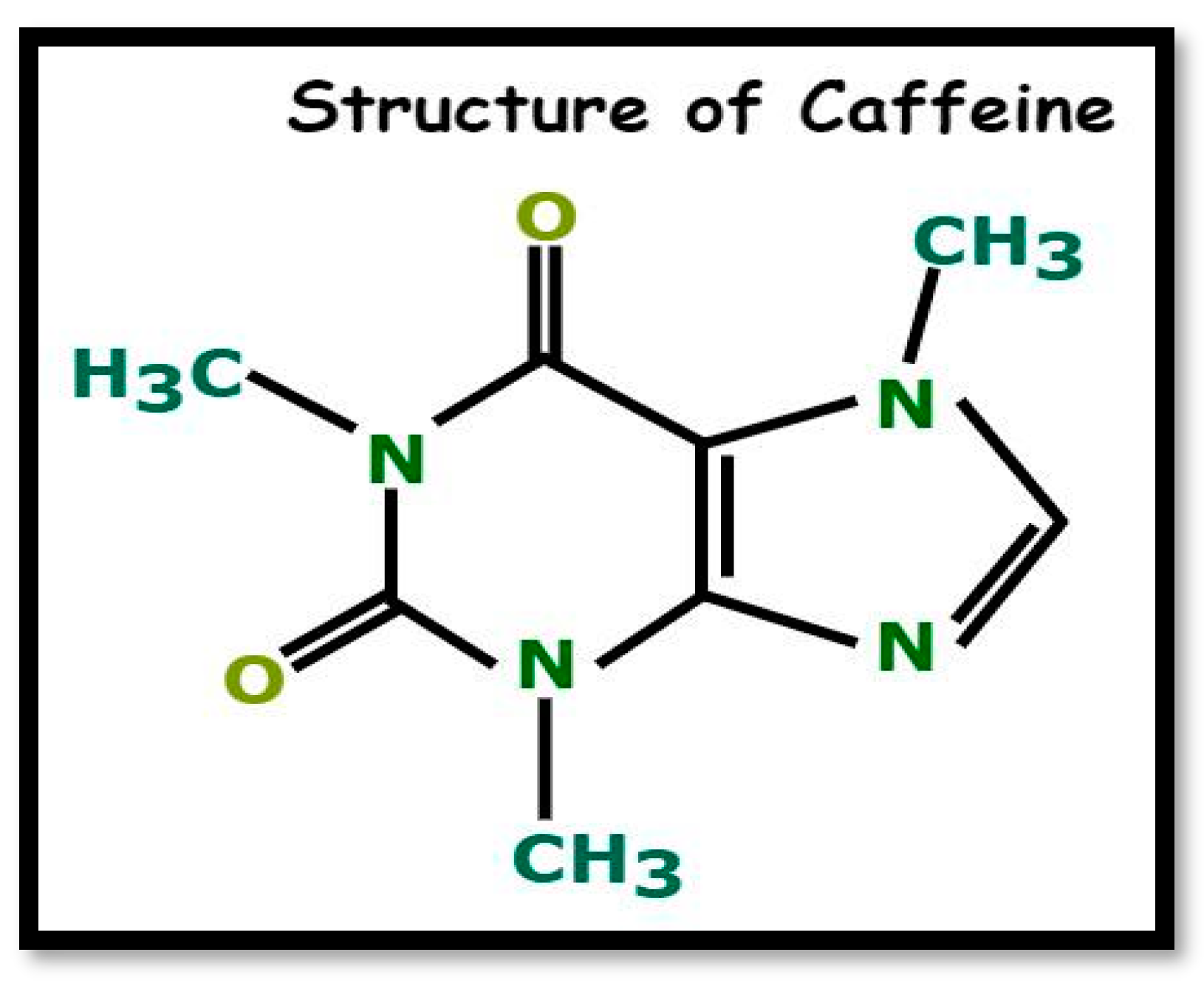
Caffeine is a naturally occurring chemical substance found in various plants and belongs to the alkaloid family, characterized by nitrogen-containing rings that exhibit biological activity and a distinctive bitter taste. It is one of the primary methyl-xanthines in tea, along with theobromine and theophylline, and exists in significant quantities with the molecular formula C8H10N4O2, commonly known as 1,3,7-trimethylxanthine (Figure 1) [1]. As a mild central nervous system stimulant, caffeine is a principal component in pharmaceuticals like No-Doze, which help enhance alertness and delay the onset of fatigue [2]. Tea leaves contain approximately 2% to 4.5% caffeine by dry weight, making them a valuable source of this compound. During the production of black tea, caffeine-rich tea waste is generated as a byproduct, following a four-step process: plucking, withering, rolling, fermentation, and firing [3].
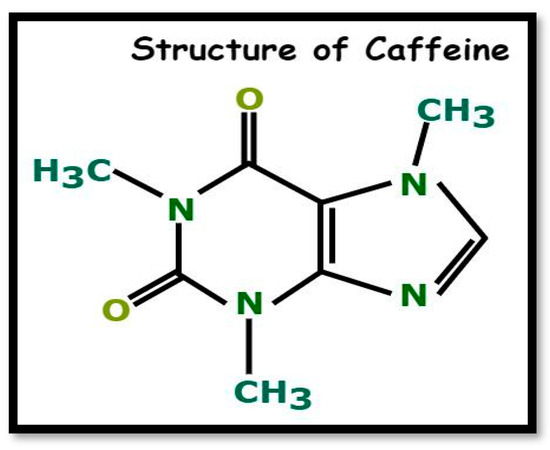
Figure 1.
Structure of caffeine.
The extraction of caffeine has been explored through various techniques, including traditional water-based extraction, supercritical CO2 extraction, and organic solvent-based extraction, each offering distinct advantages and limitations. Water-based extraction is environmentally favorable and frequently used for decaffeination. However, it can lead to the co-extraction of other water-soluble compounds, necessitating further purification steps to achieve a high-purity caffeine product [4]. Supercritical CO2 extraction, on the other hand, is a popular solvent-free approach that uses CO2 under high pressure and temperature. It provides a selective and efficient extraction method, limiting contamination and enabling the more precise separation of caffeine from other plant constituents. Despite its effectiveness, supercritical CO2 extraction is expensive due to its need for specialized equipment and high operational costs, restricting its applicability to smaller, high-value applications [5]. Organic solvent-based caffeine extraction offers high efficiency, enabling the rapid and selective removal of caffeine from tea leaves. It is cost-effective and can be easily scaled for industrial decaffeination processes. This method ensures a high caffeine yield with a minimal loss of other valuable compounds [6]. These solvents, such as dichloromethane, ethyl acetate, acetone, hexane, chloroform, and methanol, are used for extracting caffeine from tea. Additionally, organic solvents are versatile and can be tailored to optimize the extraction conditions based on the type of tea [7].
Among the organic solvents, dichloromethane has been extensively employed for caffeine extraction due to its high affinity for caffeine molecules and efficient separation capabilities, high efficiency and low boiling point, which allows for easy recovery and reuse in the extraction process, making it a cost-effective choice for industrial applications [8]. Additionally, dichloromethane is capable of extracting caffeine rapidly, reducing the processing time compared to other solvents. These factors contribute to its widespread use in the caffeine extraction industry [9]. Previous research suggests that dichloromethane extraction can effectively yield high-purity caffeine when key parameters such as the temperature and residence time are optimized, making it a competitive option in the caffeine extraction landscape [10].
The objective of this research is to optimize the extraction of caffeine from different tea types using dichloromethane as an organic solvent. The study aims to systematically evaluate the efficiency of caffeine extraction across various tea samples, including virgin black tea, used black tea, virgin green tea and used green tea, by analyzing parameters such as the extraction time and temperature. By identifying the most effective conditions for the maximum yield and purity of caffeine, this research seeks to contribute valuable insights for both academic and industrial applications, including the development of decaffeination processes and the utilization of caffeine in pharmaceuticals and food products.
2. Material and Methodology
2.1. Materials
Tea samples were purchased from commercial shops in Hyderabad, Sindh, Pakistan. Dichloromethane, sodium carbonate, sodium sulphate and distilled water were purchased from Al- Beruni Scientific Store, Hyderabad.
2.2. Caffeine Extraction
Two separate beakers were used for approximately 8 g of two different types of tea (both green and black tea) in both used and fresh forms; these were put in each beaker separately (Figure 2). Then, 100 mL of distilled water and 4.87 g of Na2CO3 were added. The mixed solution was heated until boiling at 100 °C for 30 min on a hot plate [11]. After boiling, the solution was filtered by using filter paper and a funnel. The obtained filtrate was cooled and shifted to another separating funnel. Approximately 15 mL of dichloromethane was added to this filtrate and then the funnel was tightly sealed by a stopper. The mixture was split into two layers; the lower layer was di-chloromethane and collected from the bottom of the funnel into the conical flask. The same process was repeated by adding another 15 mL of dichloromethane into the funnel. An anhydrous sodium sulfate mixture was added to a conical flask containing the mixture of dichloromethane and held for ten minutes. After the required time, the mixer was separated with the help of filter paper and a funnel. After the filtration process, the filtrate was weighed as the initial weight. The mixture was placed in a water bath for the evaporation of dichloromethane. A powder of caffeine in a light green color was visible when the dichloromethane was evaporated. The weight of this green-colored caffeine powder was the final weight. To calculate the total quantity of caffeine extracted, the initial weight was subtracted from the final weight.

Figure 2.
Extraction of caffeine from different types of teas using dichloromethane.
3. Result and Discussion
3.1. Effect of Temperature
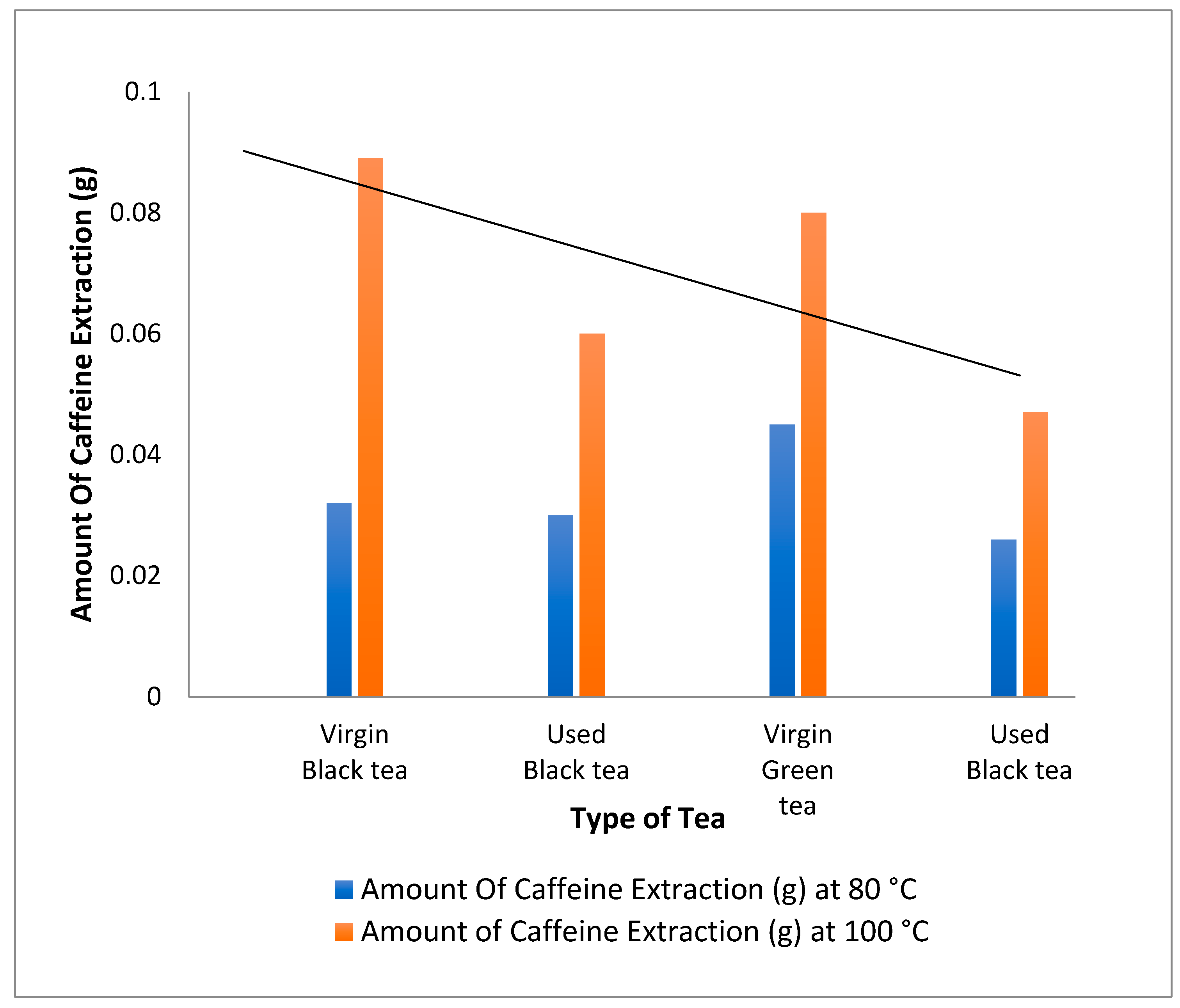
The effect of temperature on the amount of caffeine extracted from black tea, green used and virgin tea was investigated by varying the temperature between 80 °C and 100 °C for 8 g of black tea, green used and virgin tea, as shown in Table 1. The effect of temperature is shown in Figure 3.

Table 1.
Effect of temperature.
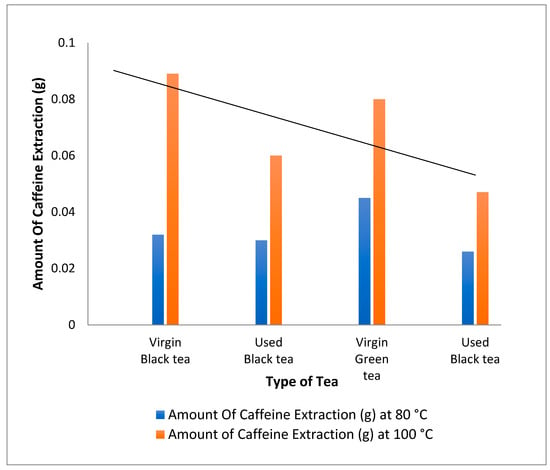
Figure 3.
Extraction of caffeine from different types of tea using dichloromethane at 80 °C and 100 °C.
3.2. Effect of Residence Time
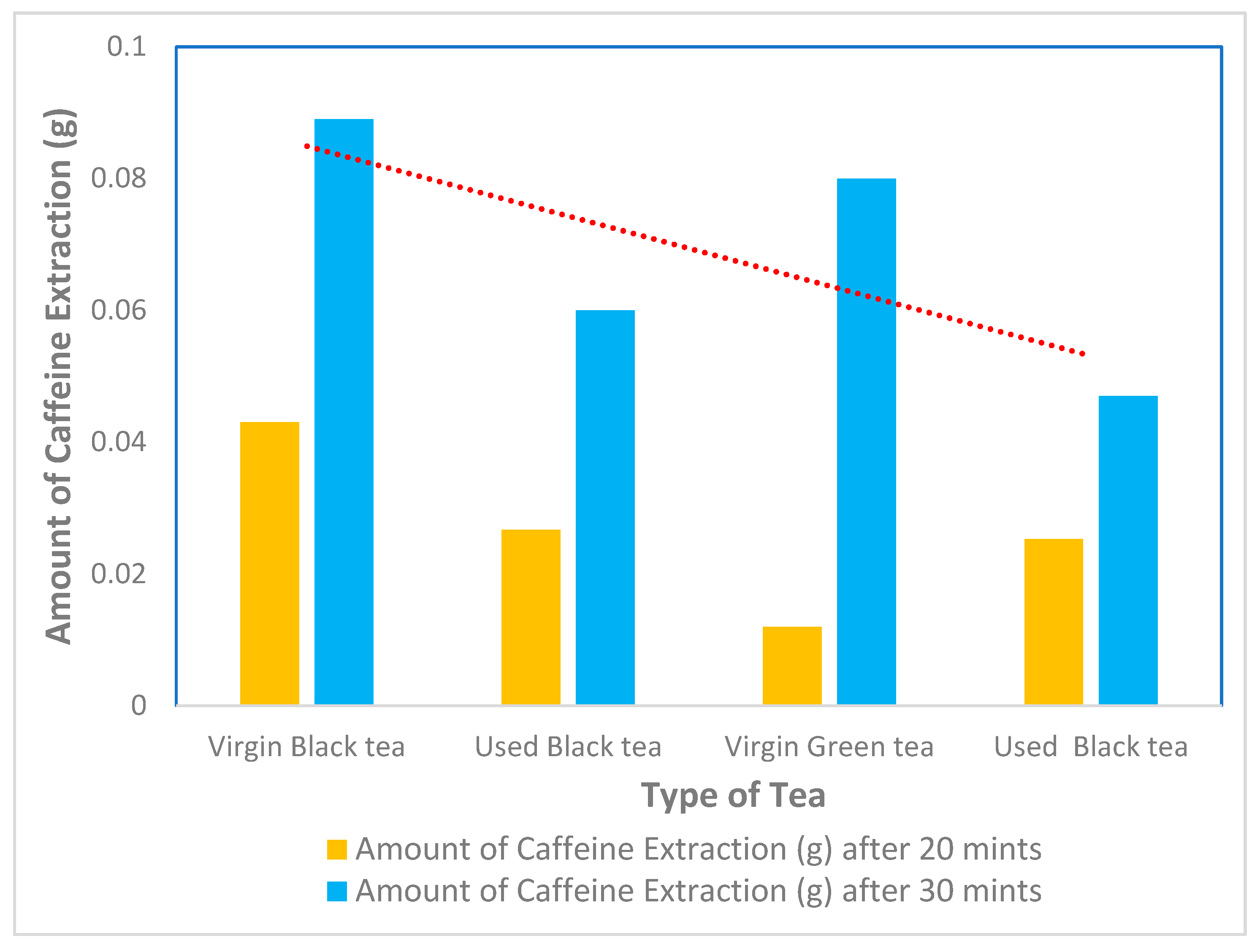
The effect of residence time on the amount of caffeine extracted from black tea, green used and virgin tea was investigated by varying the time between 20 min and 30 min at a temperature of 100 °C for 8 g of black tea, green used and virgin tea, as shown in Table 2; the effect of temperature is shown in Figure 4. The selection of 100 °C as the optimal temperature was based on both preliminary experiments and data in the literature, which suggest that higher temperatures enhance the solubility of caffeine and the extraction efficiency. This temperature was found to maximize the caffeine yield without significant degradation. Dichloromethane was chosen due to its high selectivity for caffeine, rapid extraction rate, and low boiling point, which allows for easy solvent recovery and reuse, making it a cost-effective choice for industrial-scale applications.

Table 2.
Effect of residence time.
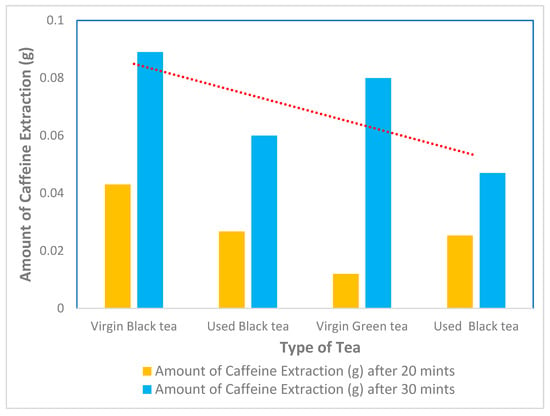
Figure 4.
Extraction of caffeine from different types of tea using dichloromethane after 20 min and 30 min.
4. Conclusions
In conclusion, this study successfully optimized the caffeine extraction process for various tea types using dichloromethane as an organic solvent. Tea, a widely consumed beverage known for its rich antioxidant content and health benefits, was selected as the source for caffeine extraction. The research focused on comparing the caffeine content in both fresh and used tea, revealing that the extracted caffeine quantities were 0.089 g from fresh black tea, 0.060 g from used black tea, 0.080 g from fresh green tea, and 0.047 g from used green tea, all extracted at 100 °C with a residence time of 30 min. These findings demonstrate that fresh tea generally yielded higher caffeine concentrations, while used tea still contained a viable amount of residual caffeine. These results underscore the potential for extracting caffeine from both fresh and used tea, depending on the optimization of the extraction parameters. This study highlights the importance of temperature and residence time in determining the extraction efficiency, with a direct correlation observed between these factors and caffeine yield. This research provides valuable insights for improving the efficiency of caffeine extraction processes, with potential applications in both industrial decaffeination and caffeine recovery from used tea.
Author Contributions
M.M.M. and M.I. contributed equally to this work. M.M.M. was responsible for conceptualization, methodology, data collection, and analysis. Both authors contributed to the investigation, validation, and interpretation of the data. Both authors participated in writing, reviewing, and editing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Depaula, J.; Farah, A. Caffeine Consumption through Coffee: Content in the Beverage, Metabolism, Health Benefits and Risks. Beverages 2019, 5, 37. [Google Scholar] [CrossRef]
- Faudone, G.; Arifi, S.; Merk, D. The Medicinal Chemistry of Caffeine. J. Med. Chem. 2021, 64, 7156–7178. [Google Scholar] [CrossRef] [PubMed]
- Patil, P. Caffeine in Various Samples and Their Analysis with HPLC—A Review. Int. J. Pharm. Sci. Rev. Res. 2012, 16, 76–83. [Google Scholar]
- Temple, J.L.; Bernard, C.; Lipshultz, S.E.; Czachor, J.D.; Westphal, J.A.; Mestre, M.A. The Safety of Ingested Caffeine: A Comprehensive Review. Front. Psychiatry 2017, 8, 80. [Google Scholar] [CrossRef] [PubMed]
- Martínez-López, S.; Sarriá, B.; Baeza, G.; Mateos, R.; Bravo-Clemente, L. Pharmacokinetics of Caffeine and Its Metabolites in Plasma and Urine after Consuming a Soluble Green/Roasted Coffee Blend by Healthy Subjects. Food Res. Int. 2014, 64, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh-Mohammadi, V.; Zamani, B.; Afsharpour, M.; Mohammadi, A. Extraction of caffeine and catechins using microwave-assisted and ultrasonic extraction from green tea leaves: An optimization study by the IV-optimal de-sign. Food Sci. Biotechnol. 2017, 26, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Sökmen, M.; Demir, E.; Alomar, S.Y. Optimization of sequential super-critical fluid extraction (SFE) of caffeine and catechins from green tea. J. Supercrit. Fluids 2018, 133, 171–176. [Google Scholar] [CrossRef]
- Senol, A.; Aydin, A. Solid–liquid extraction of caffeine from tea waste using battery type extractor: Process optimization. J. Food Eng. 2006, 75, 565–573. [Google Scholar] [CrossRef]
- Gigliobianco, M.R.; Campisi, B.; Vargas Peregrina, D.; Censi, R.; Khamitova, G.; Angeloni, S.; Di Martino, P. Optimization of the extraction from spent coffee grounds using the desirability approach. Antioxidants 2020, 9, 370. [Google Scholar] [CrossRef] [PubMed]
- Román-Montalvo, D.; Sánchez, A.; Lorenzana-Licea, E.; Domínguez, Z.; Matus, M.H. Extraction of caffeine from coffee husk employing choline-based ionic liquids: Optimization of the process and theoretical study on solute-salts interactions. J. Mol. Liq. 2024, 398, 124286. [Google Scholar] [CrossRef]
- Capuci, A.P.S.; Silva, A.C.B.; Malagoni, R.A.; Ribeiro, E.J.; Finzer, J.R.D. Extraction and Crystallization of Caffeine from Coffee Husks (Coffea Arabica Var. Catuaí). Waste Biomass Valor. 2024, 15, 4947–4963. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).