Incorporation and Mobilisation of Health-Related Organisms from within Drinking Water Biofilm †
Abstract
:1. Introduction
2. Materials and Methods
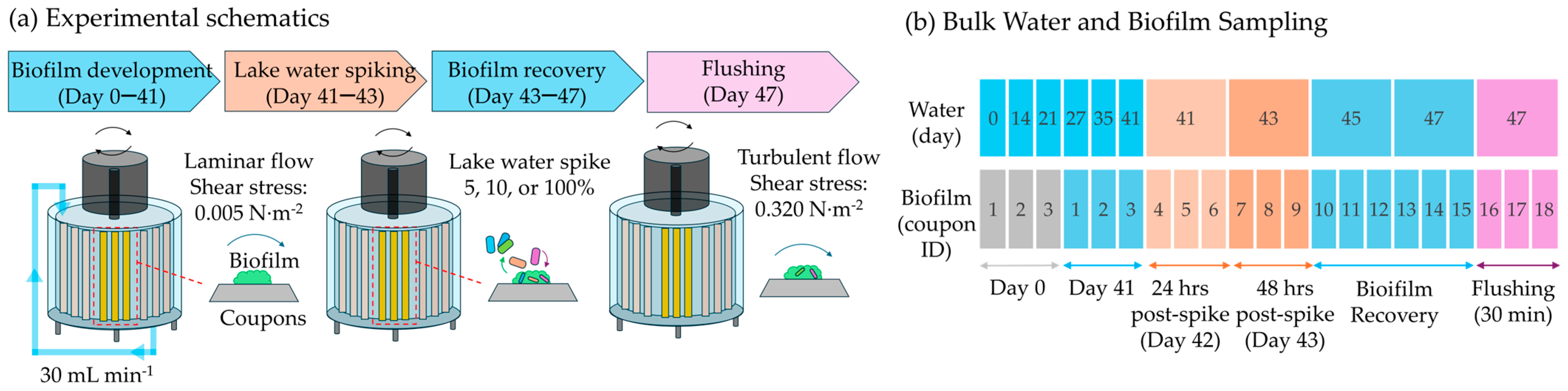
2.1. Experimental Set-Up
2.2. Bulk Water and Biofilm Sample Analysis
3. Results and Discussions
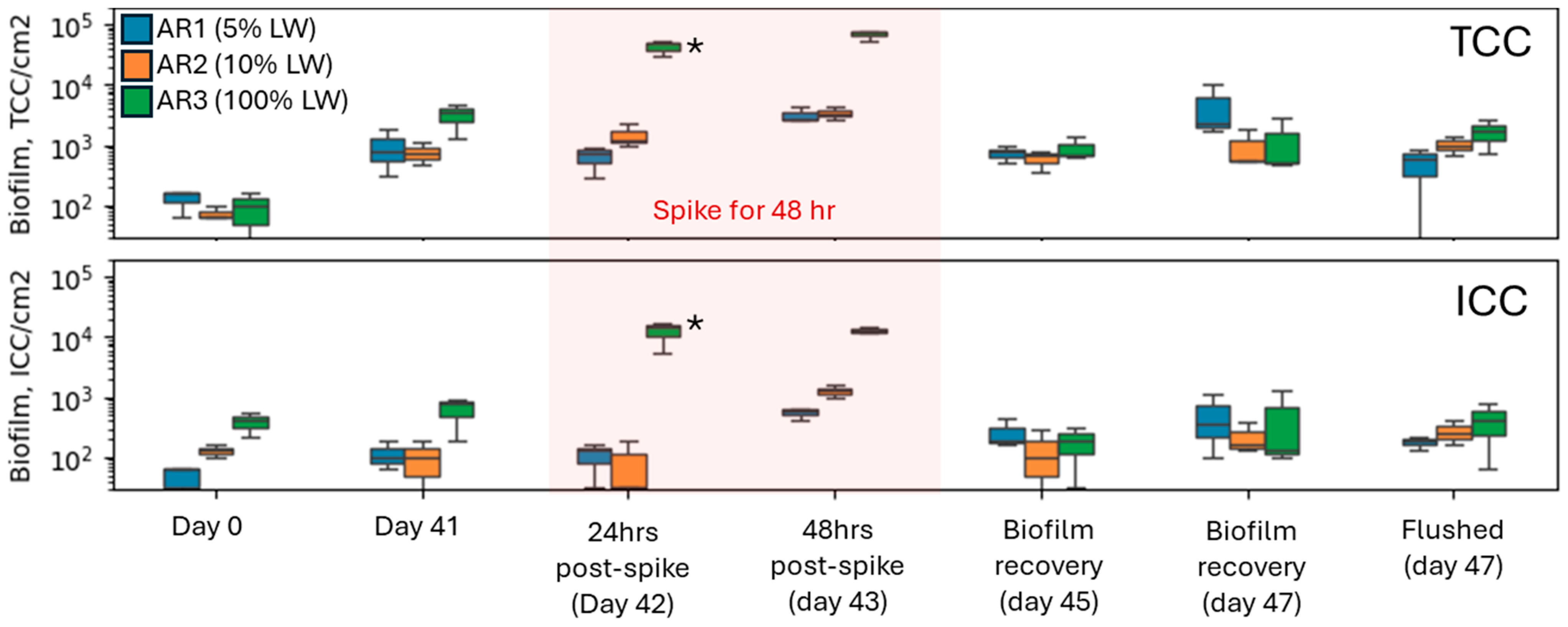
3.1. Bulk Water Quality and Biofilm Formation
3.2. Coliform Spike and Incorporation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wingender, J.; Flemming, H.C. Biofilms in drinking water and their role as reservoir for pathogens. Int. J. Hyg. Environ. Health 2011, 214, 417–423. [Google Scholar] [CrossRef]
- Gomes, I.B.; Simões, M.; Simões, L.C. An overview on the reactors to study drinking water biofilms. Water Res. 2014, 62, 63–87. [Google Scholar] [CrossRef] [PubMed]
- Fish, K.E.; Boxall, J.B. Biofilm Microbiome (Re)Growth Dynamics in Drinking Water Distribution Systems Are Impacted by Chlorine Concentration. Front. Microbiol. 2018, 9, 2519. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Wu, C.; Zhong, D.; Yuan, Y.; Shan, L.; Zhang, J. Effects of pipe materials on chlorine-resistant biofilm formation under long-term high chlorine level. Appl. Biochem. Biotechnol. 2014, 173, 1564–1578. [Google Scholar] [CrossRef] [PubMed]
- Tsagkari, E.; Sloan, W. The Role of Chlorine in the Formation and Development of Tap Water Biofilms under Different Flow Regimes. Microorganisms 2023, 11, 2680. [Google Scholar] [CrossRef] [PubMed]
- Saur, T.; Morin, E.; Habouzit, F.; Bernet, N.; Escudie, R. Impact of wall shear stress on initial bacterial adhesion in rotating annular reactor. PLoS ONE 2017, 12, e0172113. [Google Scholar] [CrossRef] [PubMed]
| Bulk Water | Inlet | AR1 | AR2 | AR3 |
|---|---|---|---|---|
| (a) TCC (×103 cells mL−1) | 3.3 ± 6.4 | 0.9 ± 0.3 | 1.5 ± 2.8 | 1.4 ± 1.1 |
| (b) ICC (×103 cells mL−1) | 1.7 ± 4.1 | 0.3 ± 0.3 | 0.7 ± 1.2 | 0.5 ± 0.5 |
| Free chlorine (mg L−1) | 0.63 ± 0.05 | 0.34 ± 0.24 | 0.34 ± 0.26 | 0.33 ± 0.23 |
| Total chlorine (mg L−1) | 0.68 ± 0.06 | 0.37 ± 0.23 | 0.39 ± 0.26 | 0.38 ± 0.24 |
| Temperature (°C) | 9.8 ± 1.0 | 12.3 ± 0.9 | 12.5 ± 0.8 | 12.5 ± 0.8 |
| Turbidity (NTU) | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| (c) ORP (mV) | 672 ± 16 | 635 ± 74 | 625 ± 87 | 643 ± 77 |
| pH | 7.43 ± 0.15 | 7.28 ± 0.06 | 7.27 ± 0.05 | 7.28 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Pick, F.; Fish, K.; Quinn, D.; Smith, C.; Speight, V.; Boxall, J. Incorporation and Mobilisation of Health-Related Organisms from within Drinking Water Biofilm. Eng. Proc. 2024, 69, 115. https://doi.org/10.3390/engproc2024069115
Park J, Pick F, Fish K, Quinn D, Smith C, Speight V, Boxall J. Incorporation and Mobilisation of Health-Related Organisms from within Drinking Water Biofilm. Engineering Proceedings. 2024; 69(1):115. https://doi.org/10.3390/engproc2024069115
Chicago/Turabian StylePark, Jiwon, Frances Pick, Katherine Fish, Dominic Quinn, Cindy Smith, Vanessa Speight, and Joby Boxall. 2024. "Incorporation and Mobilisation of Health-Related Organisms from within Drinking Water Biofilm" Engineering Proceedings 69, no. 1: 115. https://doi.org/10.3390/engproc2024069115







