Biological Treatment of Second Cheese Whey Using Marine Microalgae/Cyanobacteria-Based Systems †
Abstract
1. Introduction
2. Materials and Methods
2.1. Set Up of Biological Treatment Using Microalgae/Cyanobacteria Cultures
2.2. Analytical Methods and Procedures
3. Results and Discussion
3.1. Removal of Nutrients
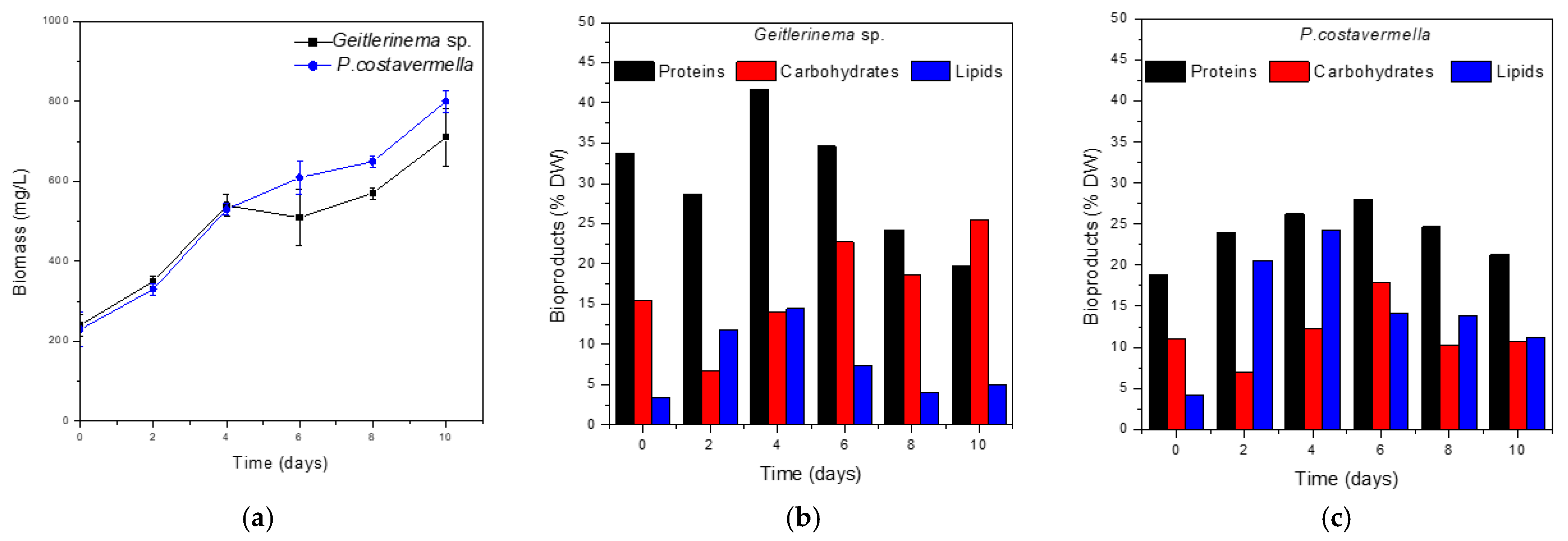
3.2. Biomass Growth and Composition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tatoulis, T.I.; Tekerlekopoulou, A.G.; Akratos, C.S.; Pavlou, S.; Vayenas, D.V. Aerobic Biological Treatment of Second Cheese Whey in Suspended and Attached Growth Reactors. J. Chem. Technol. Biotechnol. 2015, 90, 2040–2049. [Google Scholar] [CrossRef]
- Joint Ministerial Decree 145116/2011; Definition of Measures, Conditions and Procedure for Wastewater Reuse. Greek Government Gazette 354B: Athens, Greece, 2011.
- Kolakovic, S.; Stefanovic, D.; Milicevic, D.; Trajkovic, S.; Milenkovic, S.; Kolakovic, S.; Andjelkovic, L. Effects of Reactive Filters Based on Modified Zeolite in Dairy Industry Wastewater Treatment process. Chem. Ind. Chem. Eng. Q. 2013, 19, 583–592. [Google Scholar] [CrossRef]
- Kotoulas, A.; Agathou, D.; Triantaphyllidou, I.E.; Tatoulis, T.I.; Akratos, C.S.; Tekerlekopoulou, A.G.; Vayenas, D.V. Zeolite as a Potential Medium for Ammonium Recovery and Second Cheese Whey Treatment. Water 2019, 11, 136. [Google Scholar] [CrossRef]
- Kushwaha, J.P.; Srivastava, V.C.; Mall, I.D. Organics Removal from Dairy Wastewater by Electrochemical Treatment and Residue Disposal. Sep. Purif. Technol. 2010, 76, 198–205. [Google Scholar] [CrossRef]
- Tchamango, S.; Nanseu-Njiki, C.P.; Ngameni, E.; Hadjiev, D.; Darchen, A. Treatment of Dairy Effluents by Electrocoagulation Using Aluminium Electrodes. Sci. Total Environ. 2010, 408, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Bae, T.-H.; Han, S.-S.; Tak, T.-M. Membrane Sequencing Batch Reactor System for the Treatment of Dairy Industry Wastewater. Process. Biochem. 2003, 39, 221–231. [Google Scholar] [CrossRef]
- Silva, J.F.; Silva, J.R.; Santos, A.D.; Vicente, C.; Dries, J.; Castro, L.M. Continuous-Flow Aerobic Granular Sludge Treatment of Dairy Wastewater. Water 2023, 15, 1066. [Google Scholar] [CrossRef]
- Vlyssides, A.G.; Tsimas, E.S.; Barampouti, E.M.P.; Mai, S.T. Anaerobic Digestion of Cheese Dairy Wastewater Following Chemical Oxidation. Biosyst. Eng. 2012, 113, 253–258. [Google Scholar] [CrossRef]
- Tremouli, A.; Antonopoulou, G.; Bebelis, S.; Lyberatos, G. Operation and Characterization of a Microbial Fuel Cell Fed with Pretreated Cheese Whey at Different Organic Loads. Bioresour. Technol. 2013, 131, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Gannoun, H.; Khelifi, E.; Bouallagui, H.; Touhami, Y.; Hamdi, M. Ecological Clarification of Cheese Whey Prior to Anaerobic Digestion in Upflow Anaerobic Filter. Bioresour. Technol. 2008, 99, 6105–6111. [Google Scholar] [CrossRef] [PubMed]
- Venetsaneas, N.; Antonopoulou, G.; Stamatelatou, K.; Kornaros, M.; Lyberatos, G. Using Cheese Whey for Hydrogen and Methane Generation in a Two-Stage Continuous Process with Alternative pH Controlling Approaches. Bioresour. Technol. 2009, 100, 3713–3717. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.H.P. Aerobic Treatment of Dairy Wastewater. Biotechnol. Tech. 1990, 4, 1–4. [Google Scholar] [CrossRef]
- Farizoglu, B.; Keskinler, B.; Yildiz, E.; Nuhoglu, A. Cheese Whey Treatment Performance of an Aerobic Jet Loop Membrane Bioreactor. Process. Biochem. 2004, 39, 2283–2291. [Google Scholar] [CrossRef]
- Comino, E.; Riggio, V.; Rosso, M. Mountain Cheese Factory Wastewater Treatment with the Use of a Hybrid Constructed Wetland. Ecol. Eng. 2011, 37, 1673–1680. [Google Scholar] [CrossRef]
- Sultana, M.; Mourti, C.; Tatoulis, T.; Akratos, C.S.; Tekerlekopoulou, A.G.; Vayenas, D.V. Effect of Hydraulic Retention Time, Temperature, and Organic Load on a Horizontal Subsurface Flow Constructed Wetland Treating Cheese Whey Wastewater. J. Chem. Technol. Biotechnol. 2016, 91, 726–732. [Google Scholar] [CrossRef]
- Khemka, A.; Saraf, M. Phycoremediation of Dairy Wastewater Coupled with Biomass Production Using Leptolyngbya sp. J. Environ. Sci. Water Resour. 2015, 4, 104–111. [Google Scholar]
- Tsolcha, O.N.; Tekerlekopoulou, A.G.; Akratos, C.S.; Bellou, S.; Aggelis, G.; Katsiapi, M.; Moustaka-Gouni, M.; Vayenas, D.V. Treatment of Second Cheese Whey Effluents Using a Choricystis -Based System with Simultaneous Lipid Production: Treatment of Second Cheese Whey Effluents with Simultaneous Lipid Production. J. Chem. Technol. Biotechnol. 2016, 91, 2349–2359. [Google Scholar] [CrossRef]
- Tsolcha, O.N.; Tekerlekopoulou, A.G.; Akratos, C.S.; Antonopoulou, G.; Aggelis, G.; Genitsaris, S.; Moustaka-Gouni, M.; Vayenas, D.V. A Leptolyngbya-Based Microbial Consortium for Agro-Industrial Wastewaters Treatment and Biodiesel Production. Environ. Sci. Pollut. Res. 2018, 25, 17957–17966. [Google Scholar] [CrossRef] [PubMed]
- Tsolcha, O.N.; Tekerlekopoulou, A.G.; Akratos, C.S.; Aggelis, G.; Genitsaris, S.; Moustaka-Gouni, M.; Vayenas, D.V. Agroindustrial Wastewater Treatment with Simultaneous Biodiesel Production in Attached Growth Systems Using a Mixed Microbial Culture. Water 2018, 10, 1693. [Google Scholar] [CrossRef]
- Kumar, A.K.; Sharma, S.; Patel, A.; Dixit, G.; Shah, E. Comprehensive Evaluation of Microalgal Based Dairy Effluent Treatment Process for Clean Water Generation and Other Value Added Products. Int. J. Phytoremediat. 2019, 21, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.I.B.; Chagas, B.M.E.; Sassi, R.; Medeiros, G.F.; Aguiar, E.M.; Borba, L.H.F.; Silva, E.P.E.; Neto, J.C.A.; Rangel, A.H.N. Mixotrophic Cultivation of Spirulina Platensis in Dairy Wastewater: Effects on the Production of Biomass, Biochemical Composition and Antioxidant Capacity. PLoS ONE 2019, 14, e0224294. [Google Scholar] [CrossRef]
- Álvarez, X.; Arévalo, O.; Salvador, M.; Mercado, I.; Velásquez-Martí, B. Cyanobacterial Biomass Produced in the Wastewater of the Dairy Industry and Its Evaluation in Anaerobic Co-Digestion with Cattle Manure for Enhanced Methane Production. Processes 2020, 8, 1290. [Google Scholar] [CrossRef]
- Patsialou, S.; Economou, C.N.; Genitsaris, S.; Hotos, G.N.; Vayenas, D.V.; Tekerlekopoulou, A.G. Growth and Biomass Composition of the Cyanobacterium geitlerinema sp. Isolated from Hypersaline Ponds Under Different Operating Conditions. Algal Res. 2024, 81, 103564. [Google Scholar] [CrossRef]
- Dritsas, P.; Asimakis, E.; Lianou, A.; Efstratiou, M.; Tsiamis, G.; Aggelis, G. Microalgae from the Ionian Sea (Greece): Isolation, Molecular Identification and Biochemical Features of Biotechnological Interest. Algal Res. 2023, 74, 103210. [Google Scholar] [CrossRef]
- Baird, R.; Eaton, A.D.; Rice, E.W.; Bridgewater, L. (Eds.) Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 2017. [Google Scholar]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Patrinou, V.; Daskalaki, A.; Kampantais, D.; Kanakis, D.C.; Economou, C.N.; Bokas, D.; Kotzamanis, Y.; Aggelis, G.; Vayenas, D.V.; Tekerlekopoulou, A.G. Optimization of Cultivation Conditions for Tetraselmis striata and Biomass Quality Evaluation for Fish Feed Production. Water 2022, 14, 3162. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Mohan, S.V.; Mohanty, K. Dairy Wastewater Treatment Using Monoraphidium sp. KMC4 and Its Potential as Hydrothermal Liquefaction Feedstock. Bioresour. Technol. 2023, 376, 128877. [Google Scholar] [CrossRef] [PubMed]
- Hemalatha, M.; Sravan, J.S.; Min, B.; Mohan, S.V. Microalgae-Biorefinery with Cascading Resource Recovery Design Associated to Dairy Wastewater Treatment. Bioresour. Technol. 2019, 284, 424–429. [Google Scholar] [CrossRef]
- Mohanty, S.S.; Mohanty, K. Production of a Wide Spectrum Biopesticide from Monoraphidium sp. KMC4 Grown in Simulated Dairy Wastewater. Bioresour. Technol. 2023, 374, 128815. [Google Scholar] [CrossRef] [PubMed]
- Kuravi, S.D.; Mohan, S.V. Mixotrophic Cultivation of Monoraphidium sp. In Dairy Wastewater Using Flat-Panel Photobioreactor and Photosynthetic Performance. Bioresour. Technol. 2022, 348, 126671. [Google Scholar] [CrossRef]
- Kiran, B.R.; Mohan, S.V. Phycoremediation Potential of Tetradesmus sp. SVMIICT4 in Treating Dairy Wastewater Using Flat-Panel Photobioreactor. Bioresour. Technol. 2022, 345, 126446. [Google Scholar] [CrossRef]
- Ouhsassi, M.; Khay, E.O.; Bouyahya, A.; El Ouahrani, A.; El Harsal, A.; Abrini, J. Evaluation of Self-Purifying Power of Cyanobacteria Pseudanabaena galeata: Case of Dairy Factory Effluents. Appl. Water Sci. 2020, 10, 181. [Google Scholar] [CrossRef]
- Daneshvar, E.; Zarrinmehr, M.J.; Hashtjin, A.M.; Farhadian, O.; Bhatnagar, A. Versatile Applications of Freshwater and Marine Water Microalgae in Dairy Wastewater Treatment, Lipid Extraction and Tetracycline Biosorption. Bioresour. Technol. 2018, 268, 523–530. [Google Scholar] [CrossRef]
- Daneshvar, E.; Zarrinmehr, M.J.; Koutra, E.; Kornaros, M.; Farhadian, O.; Bhatnagar, A. Sequential Cultivation of Microalgae in Raw and Recycled Dairy Wastewater: Microalgal Growth, Wastewater Treatment and Biochemical Composition. Bioresour. Technol. 2019, 273, 556–564. [Google Scholar] [CrossRef]
- Bhatt, A.; Khanchandani, M.; Rana, M.S.; Prajapati, S.K. Techno-Economic analysis of microalgae cultivation for commercial sustainability: A state-of-the-art review. J. Clean. Prod. 2022, 370, 133456. [Google Scholar] [CrossRef]
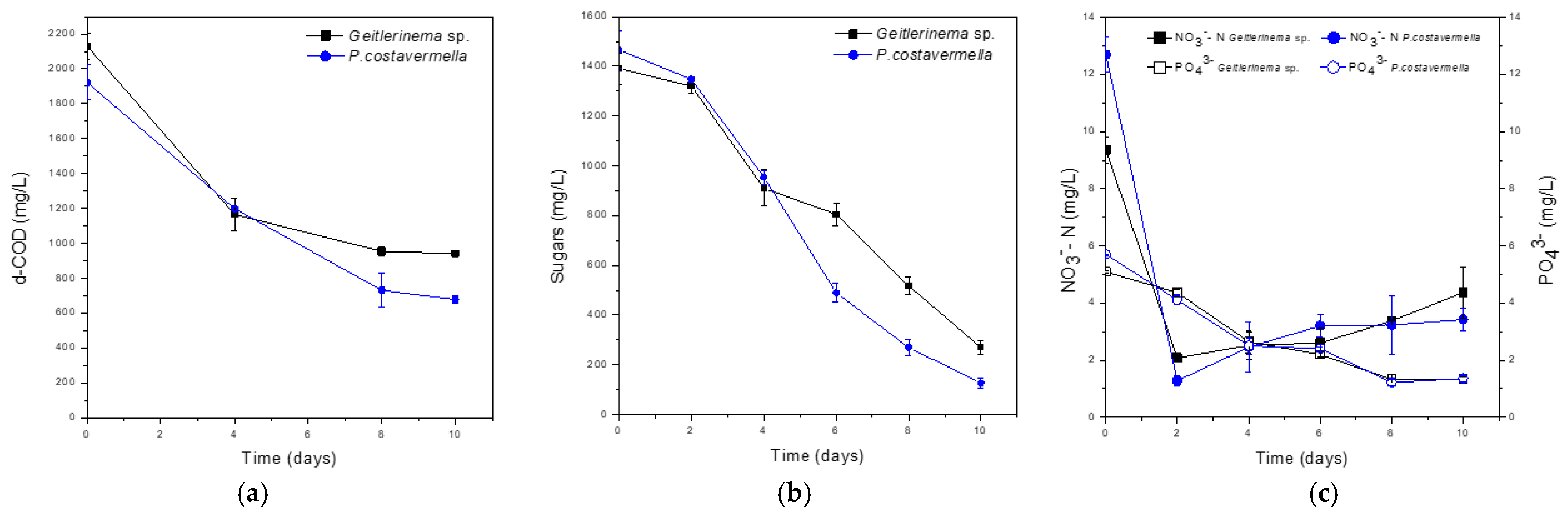
| Removal (%) | |||||
|---|---|---|---|---|---|
| d-COD | Sugars | NO3− -N | PO43− | TKN | |
| Geitlerinema sp. | 55.8 | 80.6 | 53.3 | 73.9 | 21.3 |
| Picochlorum costavermella | 64.8 | 91.4 | 73.1 | 76.4 | 45.8 |
| Biomass Concentration (mg/L) | Biomass Productivity (mg/L day) | Bioproducts (% DW) | |||
|---|---|---|---|---|---|
| Proteins | Carbohydrates | Lipids | |||
| Geitlerinema sp. | 710 | 47 | 19.8 | 25.4 | 5.0 |
| Picochlorum costavermella | 800 | 57 | 21.3 | 10.7 | 11.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patsialou, S.; Tsakona, I.A.; Vayenas, D.V.; Tekerlekopoulou, A.G. Biological Treatment of Second Cheese Whey Using Marine Microalgae/Cyanobacteria-Based Systems. Eng. Proc. 2024, 81, 4. https://doi.org/10.3390/engproc2024081004
Patsialou S, Tsakona IA, Vayenas DV, Tekerlekopoulou AG. Biological Treatment of Second Cheese Whey Using Marine Microalgae/Cyanobacteria-Based Systems. Engineering Proceedings. 2024; 81(1):4. https://doi.org/10.3390/engproc2024081004
Chicago/Turabian StylePatsialou, Stefania, Ioanna Aikaterini Tsakona, Dimitris V. Vayenas, and Athanasia G. Tekerlekopoulou. 2024. "Biological Treatment of Second Cheese Whey Using Marine Microalgae/Cyanobacteria-Based Systems" Engineering Proceedings 81, no. 1: 4. https://doi.org/10.3390/engproc2024081004
APA StylePatsialou, S., Tsakona, I. A., Vayenas, D. V., & Tekerlekopoulou, A. G. (2024). Biological Treatment of Second Cheese Whey Using Marine Microalgae/Cyanobacteria-Based Systems. Engineering Proceedings, 81(1), 4. https://doi.org/10.3390/engproc2024081004








