Abstract
Personalized medicine is an emerging field in healthcare which aims to tailor the drug and its delivery in an individualized approach to optimizing its therapeutic outcomes while minimizing its side effects. Several techniques have been developed to achieve controlled drug release. The two main technologies include active and passive devices. While passive devices can deliver the drug within a given period, they lack controllability. Active delivery devices, on the other hand, can be externally controlled in both time and drug volume. Besides this, they can be used as wearables, representing a step forward in the development of advanced drug delivery systems. In this paper, we present the development of an externally triggered active drug delivery system with the potential to be used in wearable devices. Our design was based on the development of a flexible magnetically triggerable resin and the use of cost-effective 3D printing technology to develop porous scaffolds containing the patient’s medication. Magnetic fields in the range from 56.3 ± 1.25 mT to 167.9 ± 2.62 mT were used to control the compression of the developed triggerable elastomers to dynamically adjust the drug release patterns. The obtained results demonstrate the precise and repeatable drug delivery dosage in the range of 20.7 ± 3.5 to 102.8 ± 21.7 µL/mm, showing that our approach can potentially be used to develop wearable drug delivery technologies, paving the way for personalized treatments and improved patient outcomes.
1. Introduction
Personalized medicine is revolutionizing healthcare by enabling treatments tailored to individual patient needs, improving the precision and effectiveness of medical treatments. A key component of this approach is the development of drug delivery systems (DDSs) capable of regulating medication release, optimizing therapeutic outcomes while minimizing side effects [1,2]. Traditional passive DDSs, such as hydrogels and liposomes, rely on environmental triggers such as temperature or pH. However, they lack from real-time control and precise adaptability, which limit their effectiveness [3].
To overcome these limitations, active drug delivery systems have emerged, providing greater control through external stimuli such as electric or magnetic fields, or light. Such systems enable real-time adjustments in drug release patterns and dosage, ensuring a personalized and precise treatment based on the patient-specific needs, improving therapeutic outcomes [4]. Wearable drug delivery systems show great promise by enabling on-demand medication release that responds to real-time physiological cues. This represents a significant advancement in personalized medicine aiding its development and integration into the patients’ daily lives, offering flexible and continuous medication management without the frequent involvement of doctors once the treatment has been defined [5].
In this paper, we present the design of an externally triggered, active wearable drug delivery system, which was achieved through the development a magnetic flexible composite resin and a cost-effective stereolithography 3D printer. We engineered magnetically porous elastomers capable of delivering precise and controlled drug release dosages in response to magnetic fields ranging from 56.3 ± 1.25 mT to 167.9 ± 2.62 mT. A proof-of-concept evaluation was conducted experimentally to assess the compression ratio and the repeatability capabilities of the developed magnetically triggered drug delivery system.
2. Materials and Methods
2.1. Materials and Equipment
In this study, we used Neodymium Iron Boron (NdFeB) microparticles having an average size of 5 µm (MQFP-B, Magnequench, Tianjin, China) to develop the magnetically loaded composite. Ferromagnetic polymers were formulated by dispersing the magnetic powders into a UV photocurable elastic resin (50–60A Shore hardness, Resione, F80, Dongguan Godsaid Technology, Shenzhen, China). The composite was loaded into a 3D printer (ELEGOO Mars Pro printer, Shenzhen, China) with a printing resolution of 47 µm on the X and Y axes, 1.25 µm on the Z axis, and a total build volume of 115 mm × 65 mm × 150 mm in the X, Y, and Z axes, respectively. A 405 nm back illuminated Liquid Crystal Display (LCD) screen with a resolution of 2560 by 1440 pixels was used for curing the composite. The scaffold was designed using Solid-Works software. As a starting point, a polymeric lattice topology was utilized, and particularly, the Uniform Body-Centered Cubic (UBCC) lattice geometry was selected due to its flexibility in achieving the maximum compression rate with low tensile forces comparable to those generated by permanent magnets [6].
Each scaffold block was printed with pure F80 UV resin, all having dimensions of 20 mm × 20 mm × 10 mm and 256-unit cells. The wall thickness was 2.5 mm, and the pore diameter was 0.45 mm. A cubic structure with dimensions of 20 mm × 20 mm × 5 mm was bonded into the top portion of the scaffold. This was fabricated using the developed resin composite containing 40 w/w% NdFeB magnetic particles. This preparation involved mixing the F80 resin for 2 min at 2500 rpm, heating to 45 °C to reduce viscosity, and gradually incorporating the magnetic powders into the resin. The mixture was mechanically stirred (ISTOYO, LevalIois-Perret, France) for 5 min, followed by vortex mixing (STUART SA8, Vernon Hills, IL, USA) for 3 min and then placed in an ultrasonic bath (DK sonic, Yorkshire, UK) at 45 °C for 15 min with the aim to ensure uniform dispersion. The mixed magnetic resin was poured into a vat for 3D printing. After the printing process was completed, the scaffold was removed from the platform and cleaned with ethanol using an ultrasonic bath. The elastomers were further cured with UV light (Elegoo Mercury plus 2, Shenzhen, China) at 405 nanometers and finally dried to obtain the final samples. The printed structures were then subjected to directional magnetization using an MA-3050 system (Jiujuok, Shenzhen, China) to produce a uniform magnetization [7].
2.2. Experimental Setup
An experimental setup was designed to assess the compression ratio and the repeatability capabilities of the developed 3D printed magnetically porous triggerable elastomers. Figure 1 shows the schematic of the drug delivery systems designed to conduct the experimental evaluation as follows:
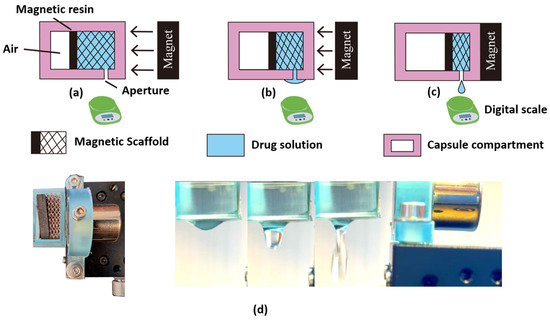
Figure 1.
Schematic diagram and representation of the magnetically controlled drug release experimental setup. (a) The uncompressed scaffold has the magnet at 10 mm. In this setting, there is no release of droplets. (b) The compressed scaffold has the magnet at 5 mm, presenting partial compression; here, the droplet release is dominated by the superficial tension of the liquid. (c) The scaffold compression has the magnet at ~0.2 mm, presenting an increased degree of compression and droplets release. The droplets are measured using the scale. (d) Experimental setup imaged using two CMOS cameras to measure both the scaffold compression (left panel) and to monitor the droplet release dynamics (right panel).
- A scaffold placed inside a 3D printed capsule compartment with a drug delivery aperture positioned at the proximal side of the enclosure. Figure 1(a—top) shows the schematic representation of the uncompressed scaffold loaded with NdFeB magnetic microparticles.
- A high precision linear stage holding the N-52-type neodymium permanent magnet (First4magnets, Nottinghamshire UK) producing a maximum magnetic field of 350 mT was manually controlled and adjusted using a micrometer stage (PT1/M, Thorlabs, Newton, NJ, USA).
- A magnetic field meter (TD8620, HFBTE, Shenzhen, China) was used to measure the magnetic field at the surface of the scaffold. The distance between the magnet and the scaffold was defined according to the measured magnetic flux with a range of 0 mm to 10 mm.
- Two CMOS cameras (FL3-U3-32S2C-CS, Teledyne FLIR, Arlington, VA, USA) were positioned above the setup to capture the scaffold’s deformation and record the changes produced in its width to calculate the scaffold’s compression ratio under different magnetic field conditions as shown in Figure 1(a–c top).
- A high precision digital scale with a resolution of 10 mg (PM6VSV Ascher, Shenzhen, China) was located below the aperture of the capsule compartment with the aim to measure the droplets released under the influence of different magnetic field strengths.
To conduct the experimental evaluation, the magnetic scaffolds were first hydrated with capillary action by immersing these into the drug solution. The scaffold was then placed inside the 3D printed capsule, having a 3 mm diameter aperture to enable the drug release. The developed 3D printed magnetic scaffold sat inside the capsule filled with the drug solution, which was held by the superficial tension created in each of the 3D printed scaffold pores.
The density of the liquid was estimated to be 1 g/mL, which was then used to estimate the released volumes. Figure 1(a–c—bottom) shows the experimental diagram of the magnetic-based drug release system, illustrating the three main stages of the experimental setup. Figure 1a—bottom shows the uncompressed scaffold having the magnet located at 10 mm. As the magnetic field increases due to the reduction of distance using the high-precision linear micrometer stage, the magnetic scaffold is compressed, as shown in Figure 1b—bottom.
The experiments revealed that the maximum compression was achieved when the magnetic field strength reached 167.9 ± 2.62 mT at ~0.2 mm, as shown in Figure 1c (left panel). It is important to mention that there was ~48% reduction of the permanent magnet’s magnetic field (350 mT) given that the capsule walls had a thickness of 2 mm. The dynamics of the droplet release are shown in Figure 1d. Here, it is observed that as the magnetic field increased, the scaffold deformed proportionally, allowing the drug to be released through the pores and capsule aperture, thus regulating its dose based on the magnetic field strength.
3. Results and Discussion
To evaluate the precision and the repeatability capabilities of the developed 3D printed magnetically porous triggerable elastomers using the experimental setup described in the previous section, two experimental tests were conducted and repeated five times:
- Assess the compression ratio of the scaffold located inside the drug delivery compartment when different magnetic field strengths are applied.
- Quantification of the mass and associated volume of the droplet released considering the density of the employed liquid.
Figure 2 shows results obtained from experiments I and II.
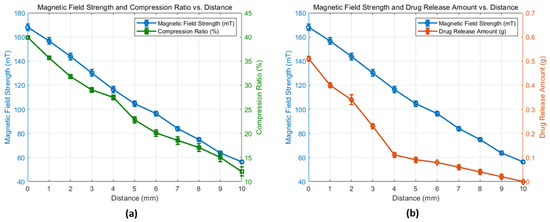
Figure 2.
(a) Experimental results of magnetic field strength versus the distance variation of the neodymium permanent magnet and the 3D printed scaffold compression ratio. (b) Experimental results of magnetic strength vs. drug release mass resulting from the distance variation between the permanent magnet and the scaffold.
Figure 2a shows the results from the experimental tests to assess the compression ratio of the scaffold, showing the relationship between magnetic field strength and the scaffold compression ratio. Such results showed that the magnetic field strength is directly proportional to the scaffold compression obtained. As the distance between the permanent magnet and the scaffold was decreased from 10 mm to 0 mm, the magnetic field strength acting on the scaffold increased from 56.3 ± 1.25 mT to 167.9 ± 2.62 mT, resulting in a compression ratio increase from 12.12 ± 0.93% to 39.90 ± 0.3%. This key finding provides reliability on the compression dynamics of the scaffold and opens the potential to further develop a predictable compression model (i.e., a linear expression), which can then be associated with the release of the liquid and therefore determine the amount of drug released as a function of the applied magnetic field with the final goal of accurately delivering a specific amount of medication to the patient.
Figure 2b shows the results from the second experiment to quantify the amount of drug release. As it is observed, it increased in a quasi-linear proportion as the magnetic field strength is increased from 56.3 ± 1.25 mT to 167.9 ± 2.62 mT. To illustrate the drug release patterns, when the magnet was positioned within the middle of the total travel range (i.e., 5 mm), the droplets released weighed 0.09 g in total; however, when the magnet was positioned at 0.2 mm from the capsule and the scaffold experienced the maximum compression, the released droplets weighed 0.51 g in total.
The obtained experimental data revealed that within the initial increase of the magnetic field strength, i.e., considering the distance from 10 to 5 mm, the release volumes were 20.7 ± 3.5 µL/mm. However, following such initial scaffold compression, the remaining liquid distribution may be held within the middle to top pores, requiring higher compression and thus higher magnetic field strengths. This resulted in the release of higher drug volumes of 102.8 ± 21.7 µL/mm. These experiments suggest that through the adjustment of the magnetic field strength, the amount of drug released can be controlled, facilitating a personalized drug delivery with the potential to be embedded in a wearable device.
4. Conclusions
In this paper, we have presented the development of an externally and magnetically triggered 3D printed scaffold embedded in a 3D printed capsule for drug delivery applications. This was conducted by formulating a novel magnetic flexible resin based on NdFeB magnetic particles, which were used to develop 3D printed porous scaffolds with a conventional 405 nm photolithographic 3D printer.
A remote triggering system was developed based on a neodymium permanent magnet, considering the decay of the magnetic field as a function of distance, producing magnetic fields in the range of 56.3 ± 1.25 mT to 167.9 ± 2.62 mT at the surface of the scaffold. A high-precision linear stage was used to control the distance and thus the variation of the magnetic field strength applied to the scaffold, which was then used to compress the developed magnetic drug delivery device, showing linear and repeatable compression. Experimental results demonstrated a system capable of delivering precise drug quantities in the range of 20.7 ± 3.5 to 102.8 ± 21.7 µL/mm. Such precise dosage control is paving the way for more effective patient-specific drug delivery solutions. This research contributes to the advancement of personalized medicine by enhancing the precision and adaptability of drug delivery technologies with the potential to be embedded in wearable devices.
Author Contributions
Conceptualization, E.R.-M.; investigation, C.Y.; methodology, E.R.-M., and R.A.-E.; project administration, E.R.-M.; hardware and data collection, C.Y.; supervision, E.R.-M., and R.A.-E.; writing, E.R.-M., R.A.-E., and C.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
The authors would like to thank the School of Information and Electronic Engineering at Zhejiang Gongshang University for their support of Chaolu Yan’s scholarship.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Timko, B.P.; Dvir, T.; Kohane, D.S. Remotely triggerable drug delivery systems. Adv. Mater. 2010, 22, 4925–4943. [Google Scholar] [CrossRef] [PubMed]
- Shademani, A.; Zhang, H.; Jackson, J.K.; Chiao, M. Active Regulation of On-Demand Drug Delivery by Magnetically Triggerable Mi-crospouters. Adv. Funct. Mater. 2017, 27, 1604558. [Google Scholar] [CrossRef]
- Shi, K.; Aviles-Espinosa, R.; Rendon-Morales, E.; Woodbine, L.; Maniruzzaman, M.; Nokhodchi, A. Novel 3D printed device with integrated macroscale magnetic field triggerable anti-cancer drug delivery system. Colloids Surf. B Biointerfaces 2020, 192, 111068. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, B.H.; Park, C.G.; Lee, C.; Lim, B.Y.; Choy, Y.B. Implantable small device enabled with magnetic actuation for on-demand and pulsatile drug delivery. J. Control. Release 2018, 286, 224–230. [Google Scholar] [CrossRef]
- Fu, Y.; Guo, Y.X. Wearable permanent magnet tracking system for wireless capsule endoscope. IEEE Sens. J. 2022, 22, 8113–8122. [Google Scholar] [CrossRef]
- Askari, G.H.; Dar, U.A.; Abid, M.; Nutkani, M.B.; Pasha, R.A.; Jamil, A. Energy absorption and compression behaviour of polymeric 3D printed lattice structures—Experimental and numerical study. In Proceedings of the 2021 International Bhurban Conference on Applied Sciences and Technologies (IBCAST), Islamabad, Pakistan, 12–16 January 2021; IEEE: Piscataway, NJ, USA, 2021; pp. 198–203. [Google Scholar] [CrossRef]
- Yang, P.; Guo, Y.; Xue, X.; Huang, B. A novel design of hard magnetic soft switch array for planar and curved surface applications. Front. Mater. 2024, 11, 1385988. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).