Quantitative Risk Assessment of Ammonia Release from Storage Tanks Using RISKCURVES Software †
Abstract
1. Introduction
2. Methods
3. Results and Discussion
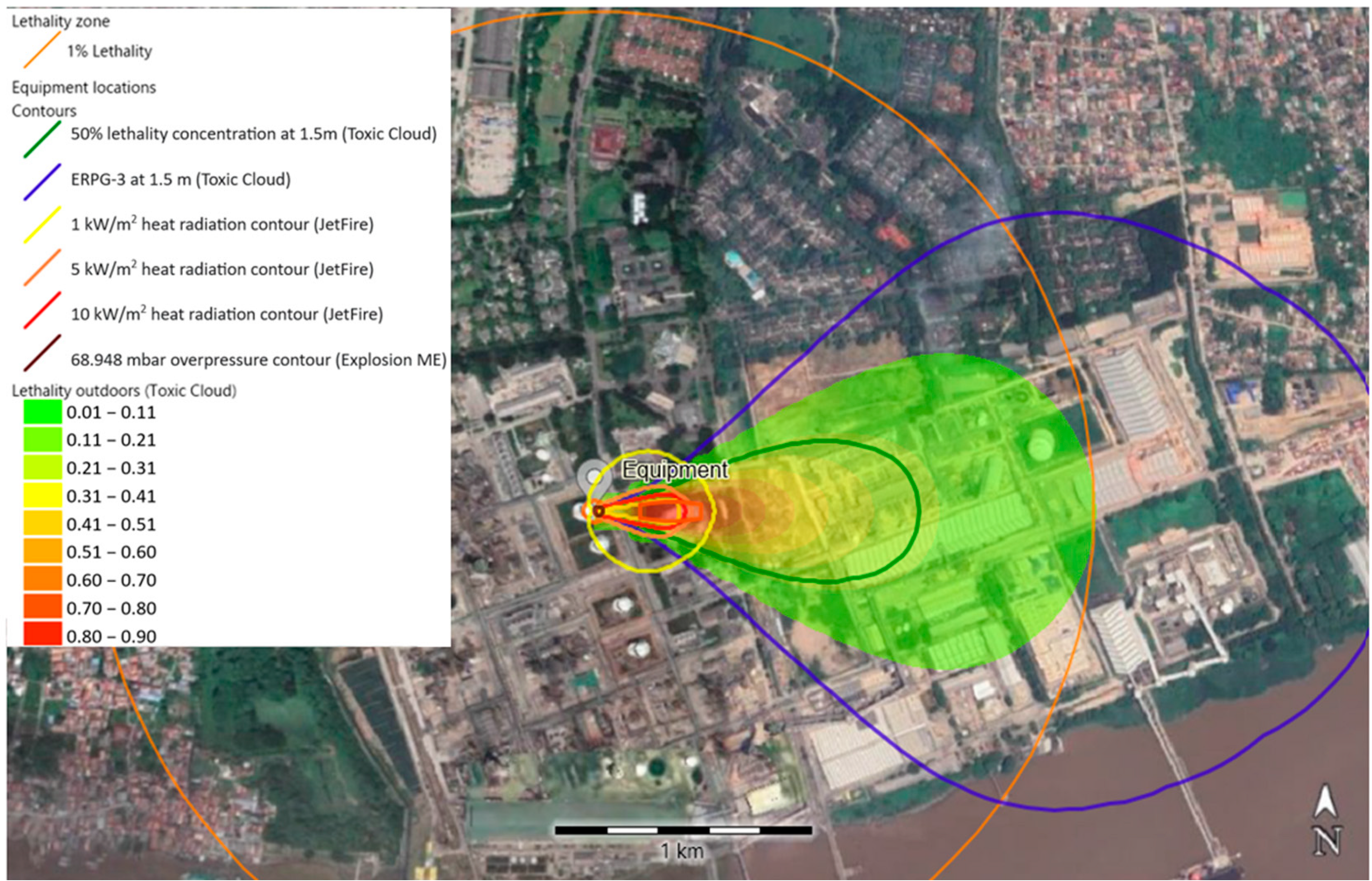
3.1. Jet Fire Simulation
3.2. Vapor Cloud Explosion (VCE) Simulations
3.3. Pool Fire and BLEVE Blast Simulation
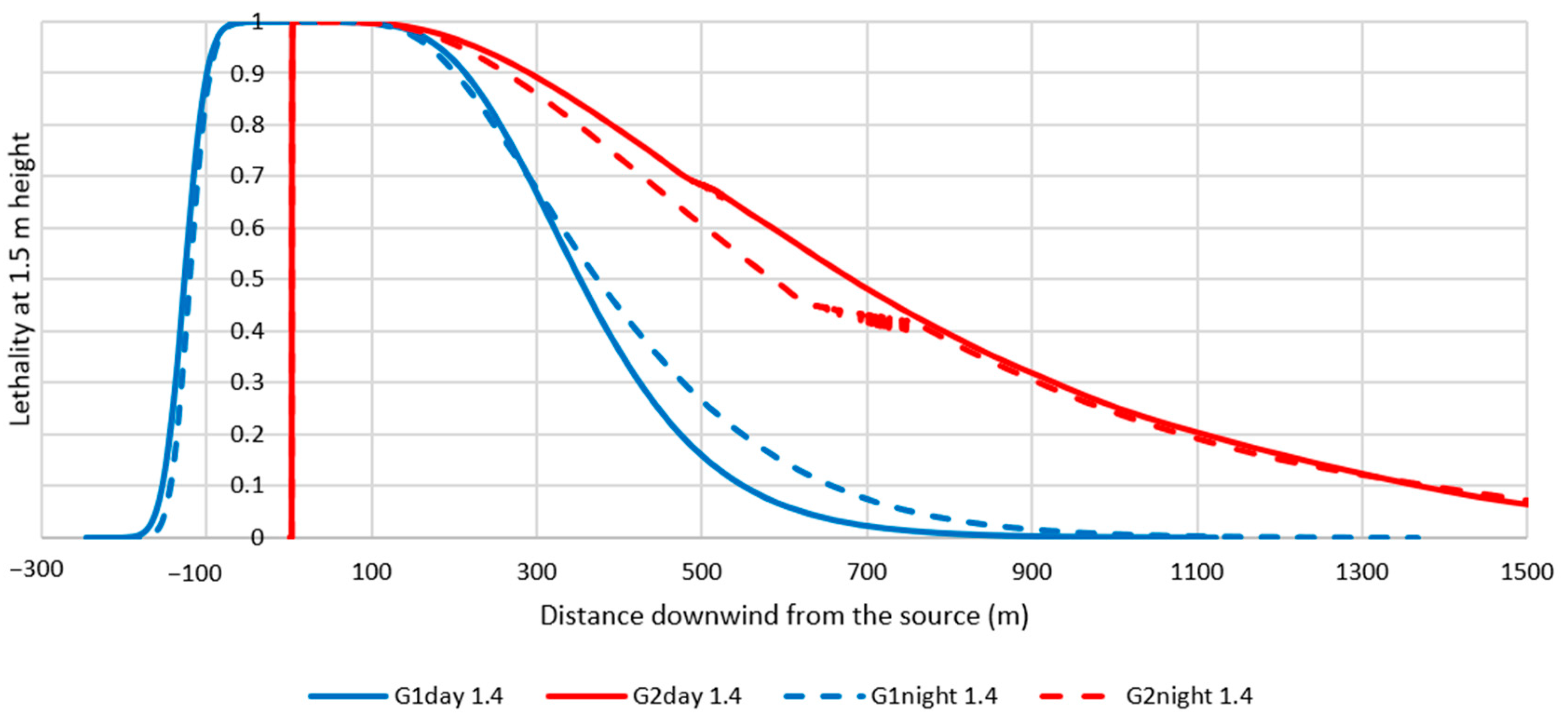
3.4. Toxic Dispersion Simulation
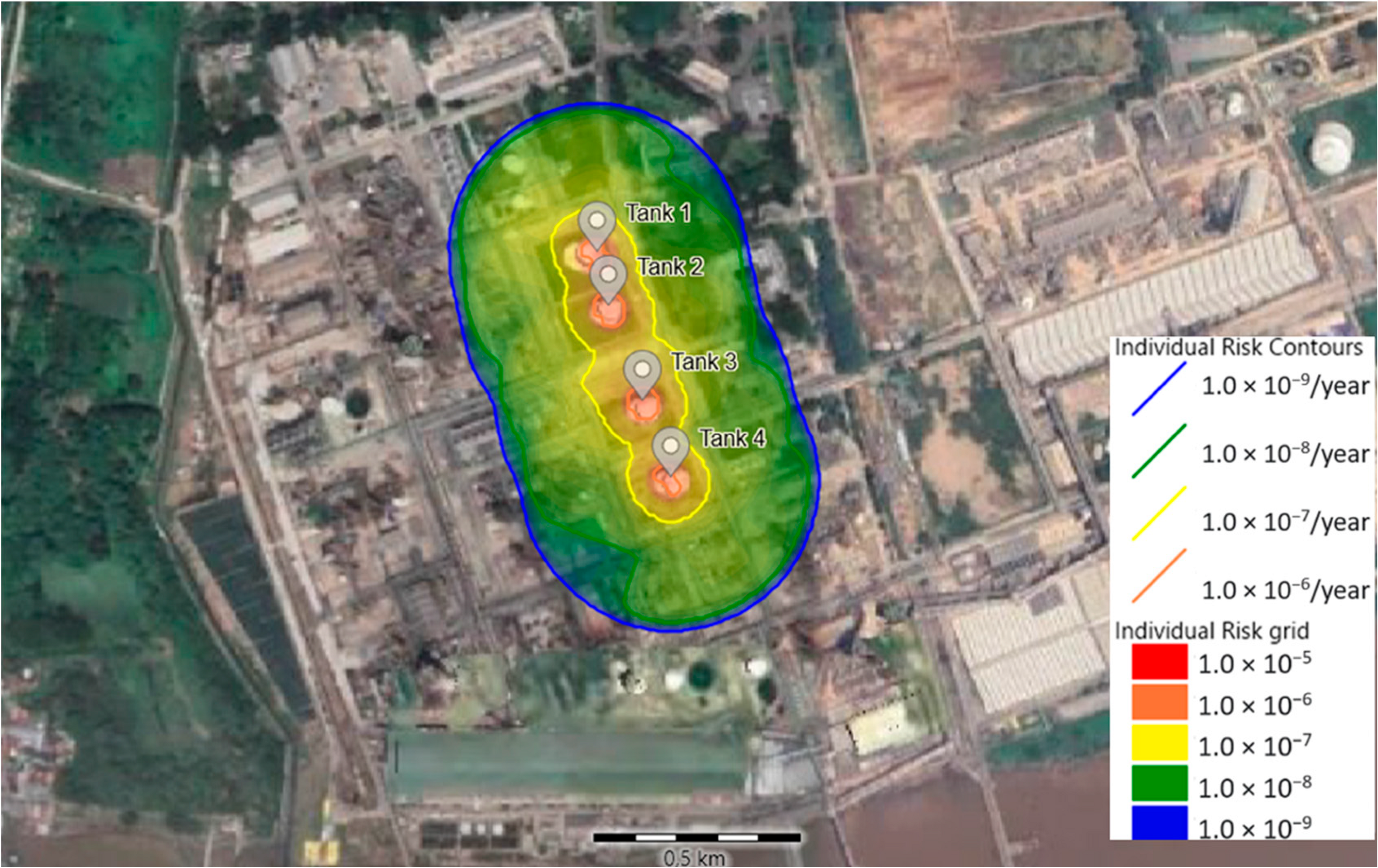
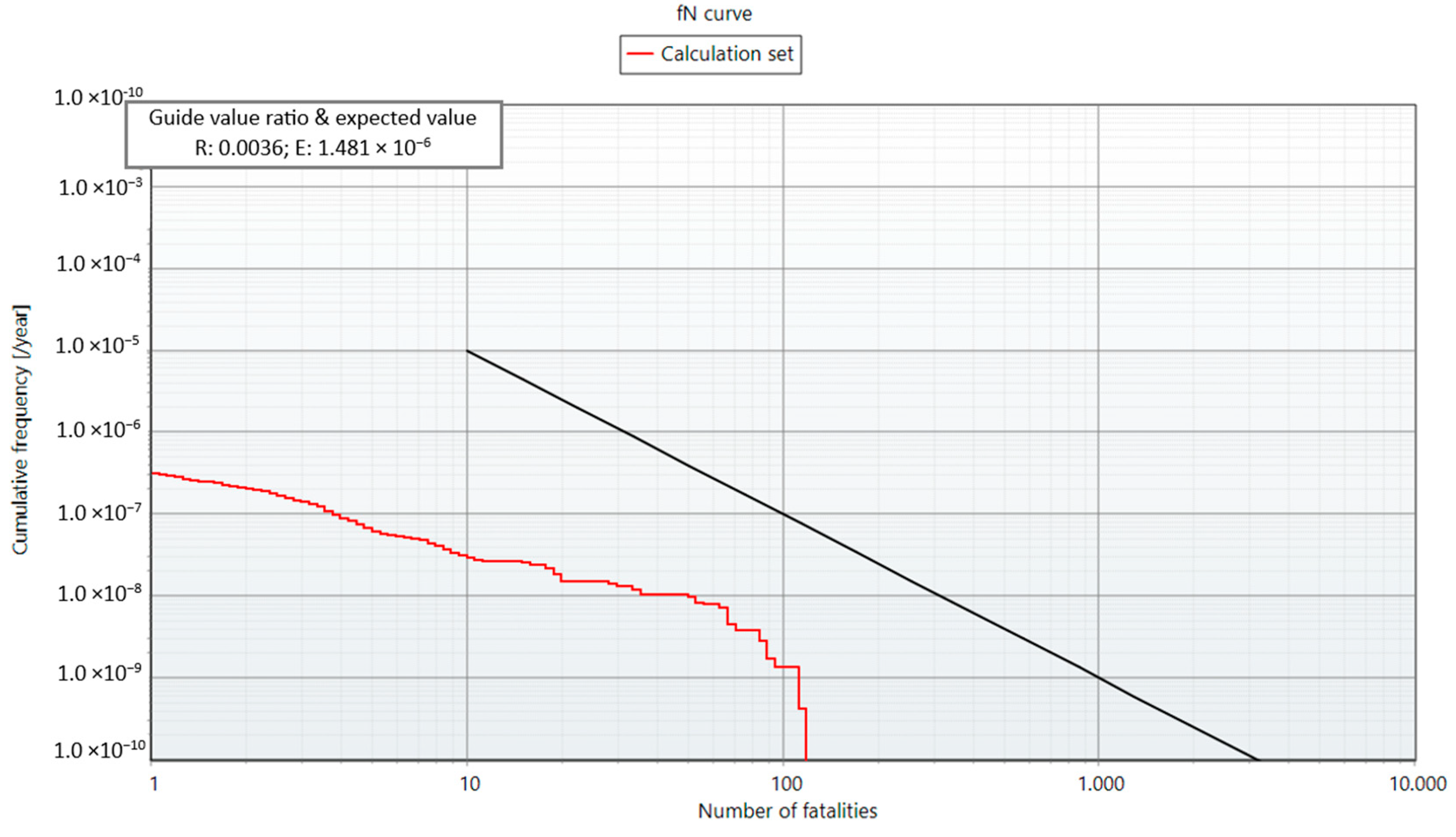
3.5. Individual and Societal Risks
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Statista Research Department Global Consumption of Agricultural Fertilizer from 1965 to 2021, by Nutrient. Available online: https://www.statista.com/statistics/438967/fertilizer-consumption-globally-by-nutrient/ (accessed on 20 September 2024).
- Apriani, M. Analisis Risiko Kegagalan Dan Basic Cause Kebocoran Pada Tangki Penyimpanan Amonia. J. Purifikasi 2016, 16, 102–110. [Google Scholar] [CrossRef][Green Version]
- U.S. EPA. Hazards of Ammonia Releases at Ammonia Refrigeration Facilities (Update) [Fact Sheet]. EPA 550-F-01-009. 2001. Available online: https://www.epa.gov/sites/default/files/2013-11/documents/ammonia.pdf (accessed on 3 March 2024).
- French Ministry of the Environment. Explosion of an Ammonia Tank March 24,1992 Dakar Senegal. 2006. Available online: https://www.aria.developpement-durable.gouv.fr/wp-content/files_mf/A3485_ips03485_002.pdf (accessed on 2 March 2024).
- Stewart, E. Revisiting the Jonava Ammonia Tank Rupture—35 Years On. Loss Prev. Bull. 2024, 295, 2–6. [Google Scholar]
- Masriadi. Kebocoran Amonia Di Aceh, PIM Akui Katup Pengaman Pabrik Terbuka. Kompas, 14 November 2016. Available online: https://regional.kompas.com/read/2016/11/14/05163831/kebocoran.amonia.di.aceh.pim.akui.katup.pengaman.pabrik.terbuka (accessed on 2 March 2024).
- Prihatini, Z.; Movanita, A.N.K. 200 Warga Dievakuasi Imbas Kebocoran Gas Amonia Di Pabrik Es Karawaci. Kompas, 6 February 2024. Available online: https://megapolitan.kompas.com/read/2024/02/06/17424901/200-warga-dievakuasi-imbas-kebocoran-gas-amonia-di-pabrik-es-karawaci (accessed on 2 March 2024).
- Nashira, A.; Rahmah, A.U.; Wahyuningsih, A.; Azizah, P.R.N. Risk Assessment of Hospital Waste Water Treatment Plant Operation—A Case Study of a Class B Hospital in Indonesia. Int. J. Saf. Secur. Eng. 2024, 14, 1305–1317. [Google Scholar] [CrossRef]
- El Harbawi, M.; Mustapha, S.; Choong, T.S.Y.; Abdul Rashid, S.; Kadir, S.A.S.A.; Abdul Rashid, Z. Rapid Analysis of Risk Assessment Using Developed Simulation of Chemical Industrial Accidents Software Package. Int. J. Environ. Sci. Technol. 2008, 5, 53–64. [Google Scholar] [CrossRef]
- Danasa, A.S.; Soesilo, T.E.B.; Martono, D.N.; Sodri, A.; Hadi, A.S.; Chandrasa, G.T. The Ammonia Release Hazard and Risk Assessment: A Case Study of Urea Fertilizer Industry in Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2019, 399, 012087. [Google Scholar] [CrossRef]
- Novrikasari, N.; Lestari, F.; Sudiman, D.R.; Kamso, S.; Nugroho, Y.S.; Prasetyo, B.T.; Wispriyono, B.; Gunawan, F.A.; Andarini, D. Analisis Kesiapsiagaan Bencana Teknologi Dari Pabrik X Pada Aspek Proyeksi Zona Bahaya. J. Kesehat. Lingkung. Indones. 2023, 22, 38–45. [Google Scholar] [CrossRef]
- de Haag, P.A.M.U.; Ale, B.J.M. Guideline for Quantitative Risk Assessment “Purple Book”; National Institute of Public Health and the Environment (RIVM): Bilthoven, The Netherlands, 2005. [Google Scholar]
- Anjana, N.S.; Amarnath, A.; Harindranathan Nair, M.V. Toxic Hazards of Ammonia Release and Population Vulnerability Assessment Using Geographical Information System. J. Environ. Manag. 2018, 210, 201–209. [Google Scholar] [CrossRef]
- Wusnah; Sylvia, N.; Bindar, Y. Kajian Pengaruh Angin Silang Terhadap Karakteristik Nyala Hidrokarbon. J. Teknol. Kim. Unimal 2013, 1, 25–37. [Google Scholar]
- Office of Response and Restoration Thermal Radiation Levels of Concern. Available online: https://response.restoration.noaa.gov/oil-and-chemical-spills/chemical-spills/resources/thermal-radiation-levels-concern.html (accessed on 20 September 2024).
- Thomas, J.K.; Malik, D.R. Ammonia and Hydrogen Vapor Cloud Explosion Testing (A Tale of Two Gases). In Proceedings of the 62nd Annual Safety in Ammonia Plants and Related Facilities Symposium, Brooklyn, NY, USA, 10–14 September 2017. [Google Scholar]
- Perwitasari, P.; Sumardi, S.; Perdana, I. Model Dispersi Gas Dan Vapor Cloud Explosion Pada Kebocoran Outlet Pigtail Tubes Primary Reformer. J. Rekayasa Proses 2018, 12, 9. [Google Scholar] [CrossRef][Green Version]
- Corruchaga, A.; Casal, O.; Palacios, A.; Casal, J. Influence of Wind Speed and Ammonia Concentration on Its Evaporation Rate from Aqueous Solution Spills. J. Loss Prev. Process Ind. 2022, 76, 104750. [Google Scholar] [CrossRef]
- UK HSE Guidance on ALARP Decisions in COMAH. Available online: https://www.hse.gov.uk/foi/internalops/hid_circs/permissioning/spc_perm_37/ (accessed on 20 September 2024).
| Parameter | Day | Night |
|---|---|---|
| Relative humidity | 67% | 92% |
| Temperature | 31 °C | 25 °C |
| Pressure | 1.009 bar | 1.007 bar |
| Average wind speed | 0.3 m/s; 1.4 m/s; 3.3 m/s | |
| G1 | G2 | G3 | |
|---|---|---|---|
| Release type | Instantaneous | Continuous, 10 min to empty | Continuous, hole 10 mm |
| Frequency | 5 × 10−7 | 5 × 10−7 | 1 × 10−5 |
| Heat Radiation Contours | Distance (m) | |||||
|---|---|---|---|---|---|---|
| Day | Night | |||||
| 0.3 m/s | 1.4 m/s | 3.3 m/s | 0.3 m/s | 1.4 m/s | 3.3 m/s | |
| 1 kW/m2 | 480 | 465 | 437 | 472 | 456 | 428 |
| 5 kW/m2 | 397 | 377 | 345 | 390 | 370 | 339 |
| 10 kW/m2 | 372 | 352 | 320 | 365 | 345 | 314 |
| 35 kW/m2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Scenario and Overpressure Contours | Distance (m) | |||||
|---|---|---|---|---|---|---|
| Day | Night | |||||
| 0.3 m/s | 1.4 m/s | 3.3 m/s | 0.3 m/s | 1.4 m/s | 3.3 m/s | |
| G1 overpressure contour (69 mbar) | 92 | 101 | 110 | 93 | 102 | 111 |
| G2 overpressure contour (69 mbar) | 42 | 35 | 27 | 43 | 34 | 27 |
| Contour Name | Distance (m) | |||||
|---|---|---|---|---|---|---|
| Day | Night | |||||
| 0.3 m/s | 1.4 m/s | 3.3 m/s | 0.3 m/s | 1.4 m/s | 3.3 m/s | |
| 1 kW/m2 (pool fire) | 140 | 142 | 145 | 140 | 142 | 145 |
| 5 kW/m2 (pool fire) | 76 | 79 | 85 | 76 | 79 | 85 |
| 10 kW/m2 (pool fire) | 60 | 62 | 69 | 60 | 62 | 69 |
| 69 mbar overpressure (BLEVE blast) | 34 | 34 | 34 | 34 | 34 | 34 |
| Concentration Contour | Distance (m) | |||||
|---|---|---|---|---|---|---|
| Day | Night | |||||
| 0.3 m/s | 1.4 m/s | 3.3 m/s | 0.3 m/s | 1.4 m/s | 3.3 m/s | |
| ERPG-2 at 1.5 m | 6330 | 10,602 | 13,655 | 6459 | 10,731 | 13,412 |
| ERPG-3 at 1.5 m | 1527 | 3188 | 4413 | 1835 | 3613 | 4742 |
| 50% lethality concentration at 1.5 m | 924 | 1257 | 1043 | 1005 | 1288 | 1039 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nashira, A.; Nugrahani, A.P.; Rahmah, A.U.; Cahyono, T. Quantitative Risk Assessment of Ammonia Release from Storage Tanks Using RISKCURVES Software. Eng. Proc. 2025, 84, 80. https://doi.org/10.3390/engproc2025084080
Nashira A, Nugrahani AP, Rahmah AU, Cahyono T. Quantitative Risk Assessment of Ammonia Release from Storage Tanks Using RISKCURVES Software. Engineering Proceedings. 2025; 84(1):80. https://doi.org/10.3390/engproc2025084080
Chicago/Turabian StyleNashira, Alimatun, Anindita Putri Nugrahani, Anisa Ur Rahmah, and Teguh Cahyono. 2025. "Quantitative Risk Assessment of Ammonia Release from Storage Tanks Using RISKCURVES Software" Engineering Proceedings 84, no. 1: 80. https://doi.org/10.3390/engproc2025084080
APA StyleNashira, A., Nugrahani, A. P., Rahmah, A. U., & Cahyono, T. (2025). Quantitative Risk Assessment of Ammonia Release from Storage Tanks Using RISKCURVES Software. Engineering Proceedings, 84(1), 80. https://doi.org/10.3390/engproc2025084080






