Mechanical Multiscale Lithium-Ion Battery Modeling for Optimized Battery Pack Design †
Abstract
1. Introduction
2. Materials and Methods
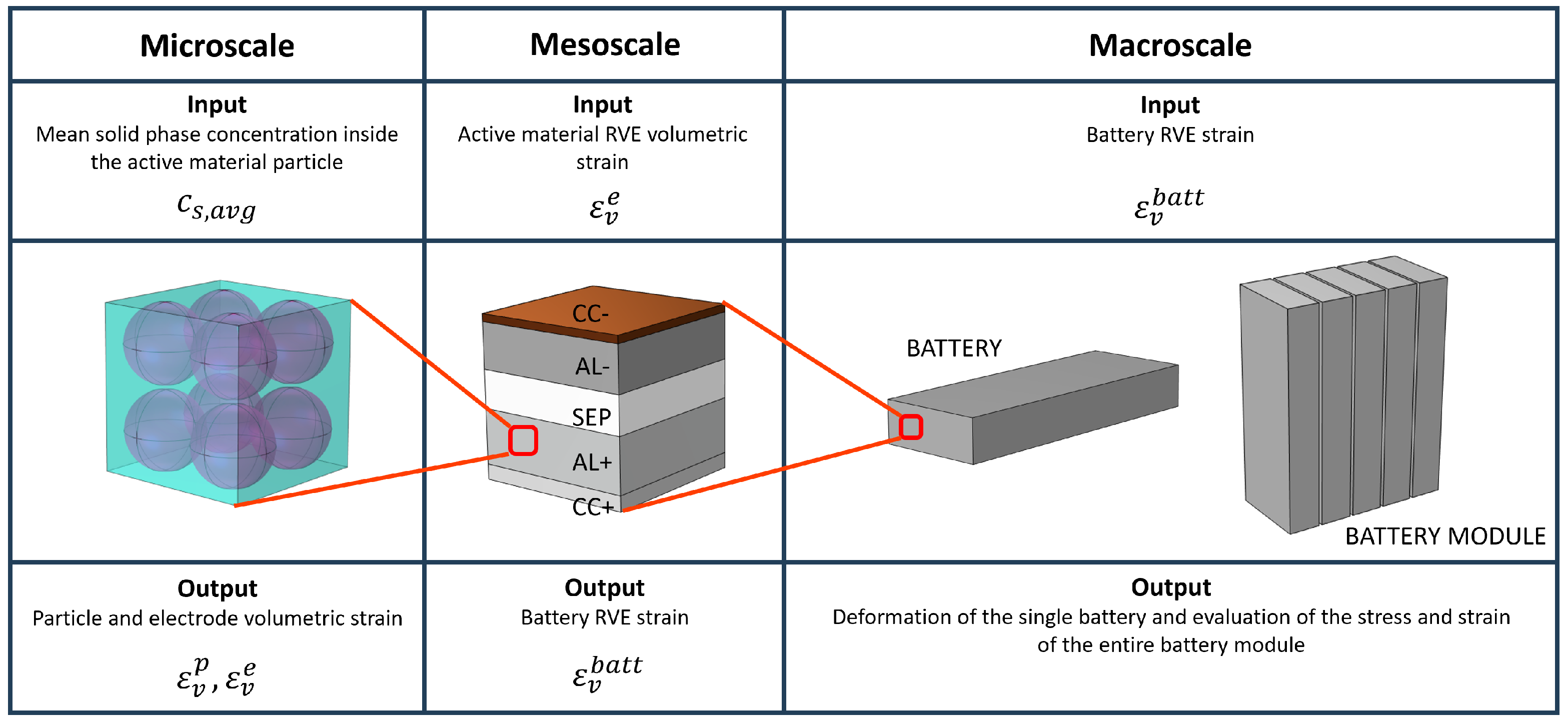
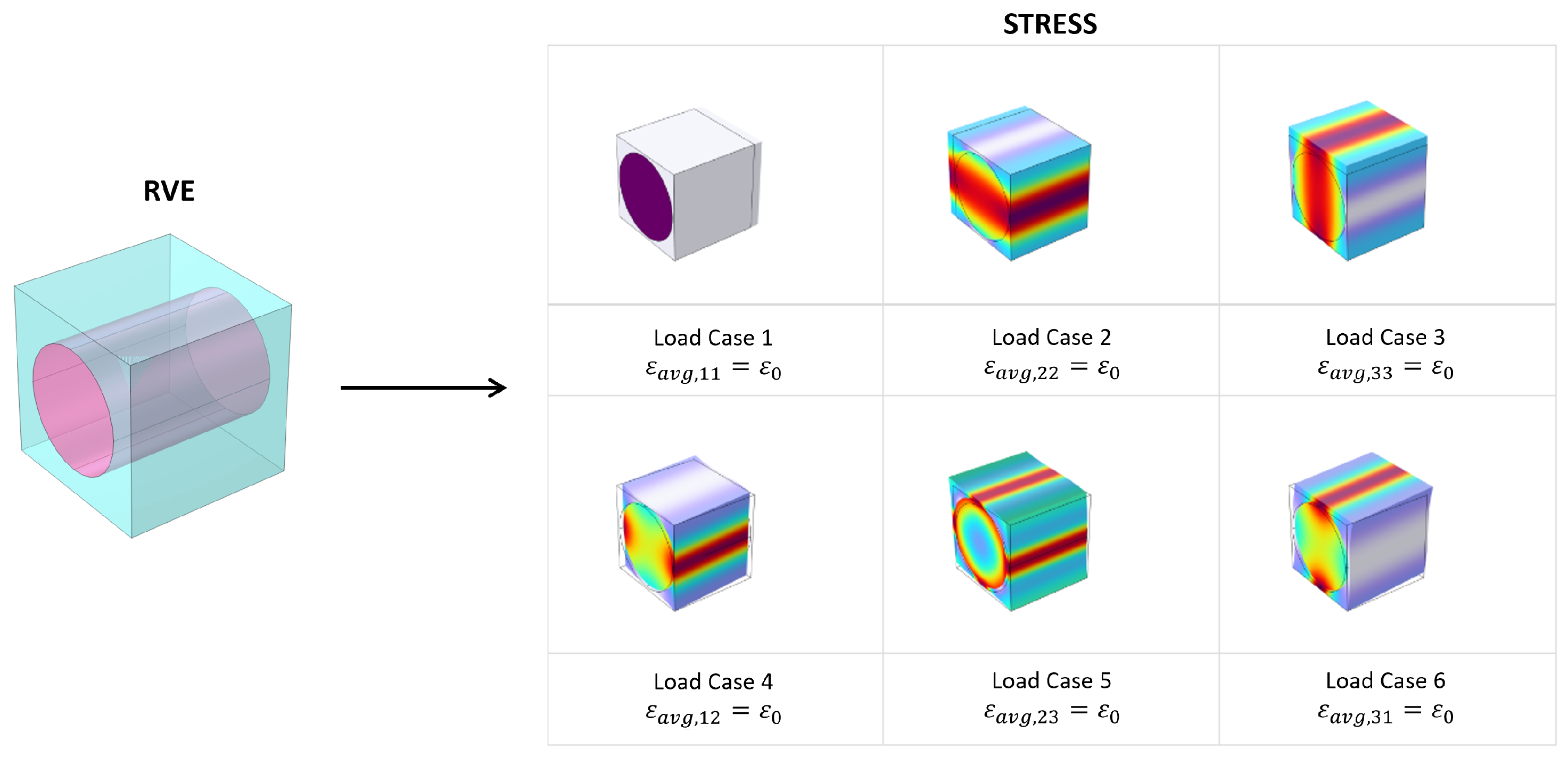
2.1. Numerical Homogenization
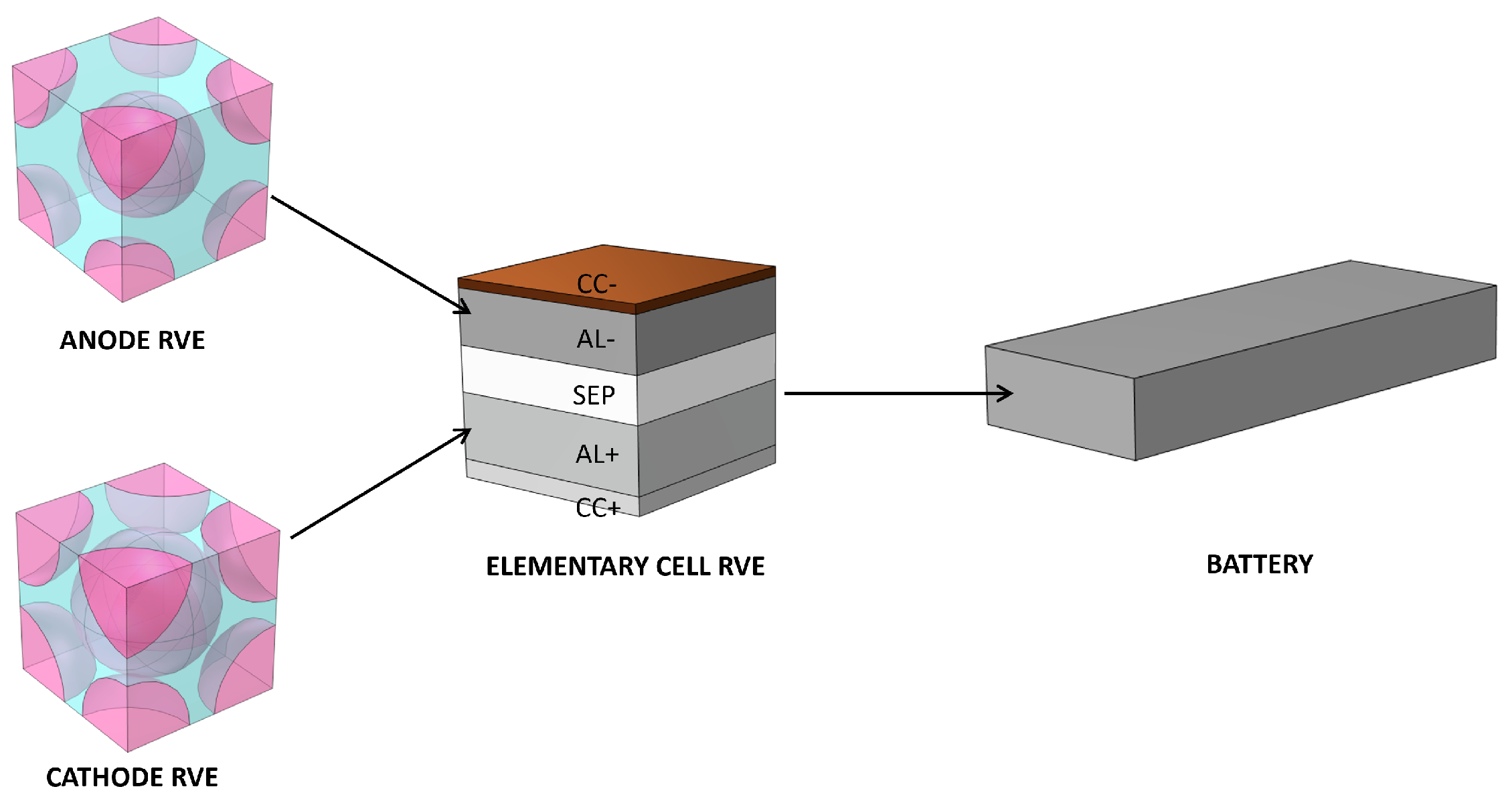
2.2. Battery Homogenization
2.3. Multiscale Model
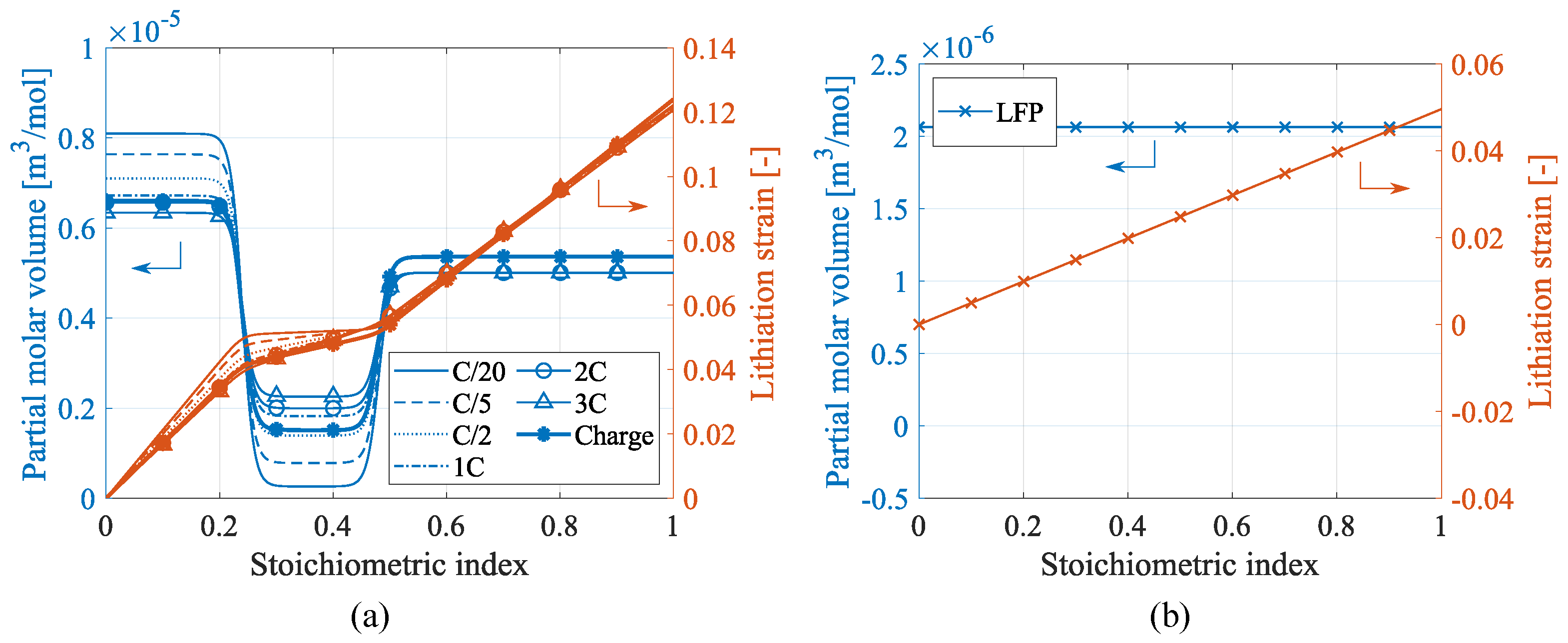
3. Experiment
4. Results
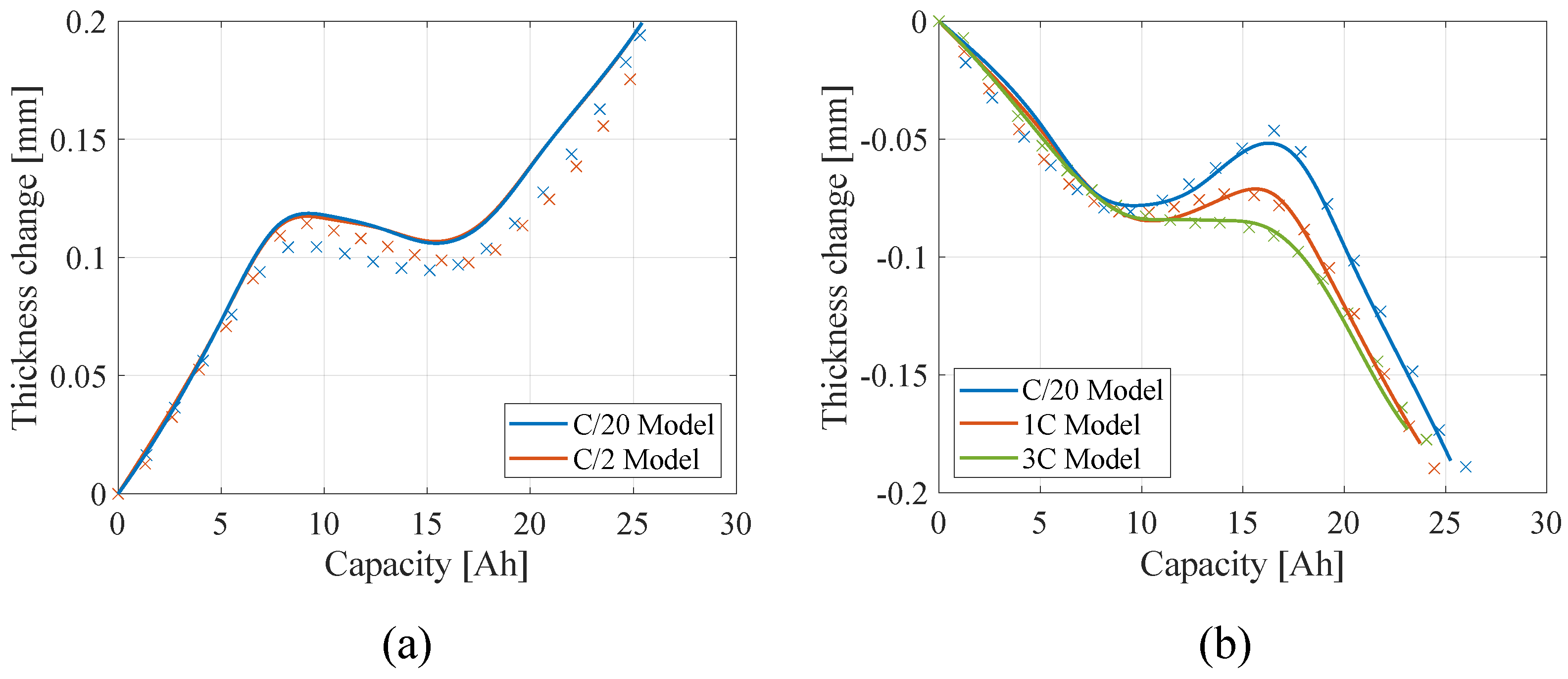
4.1. Single Battery Level
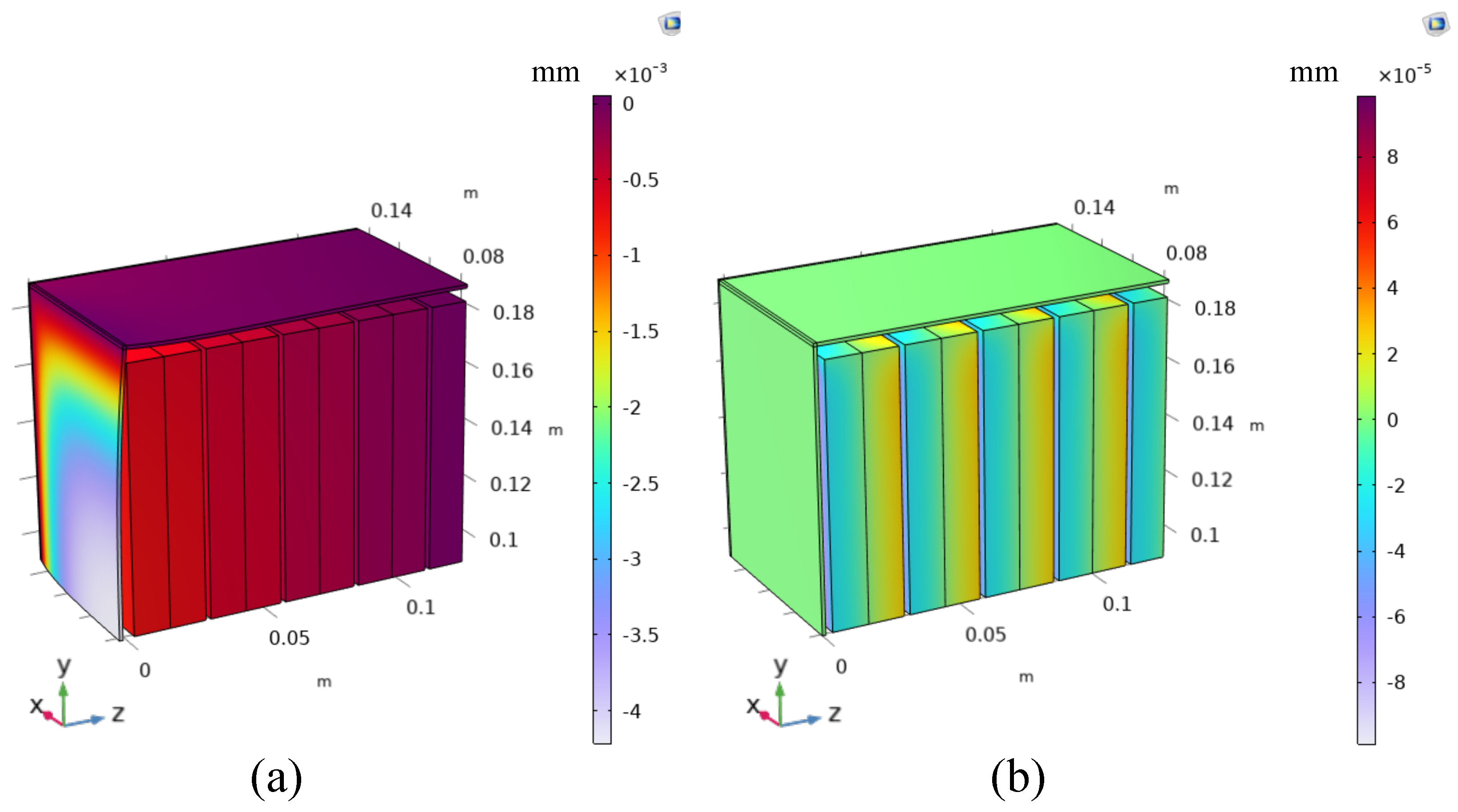
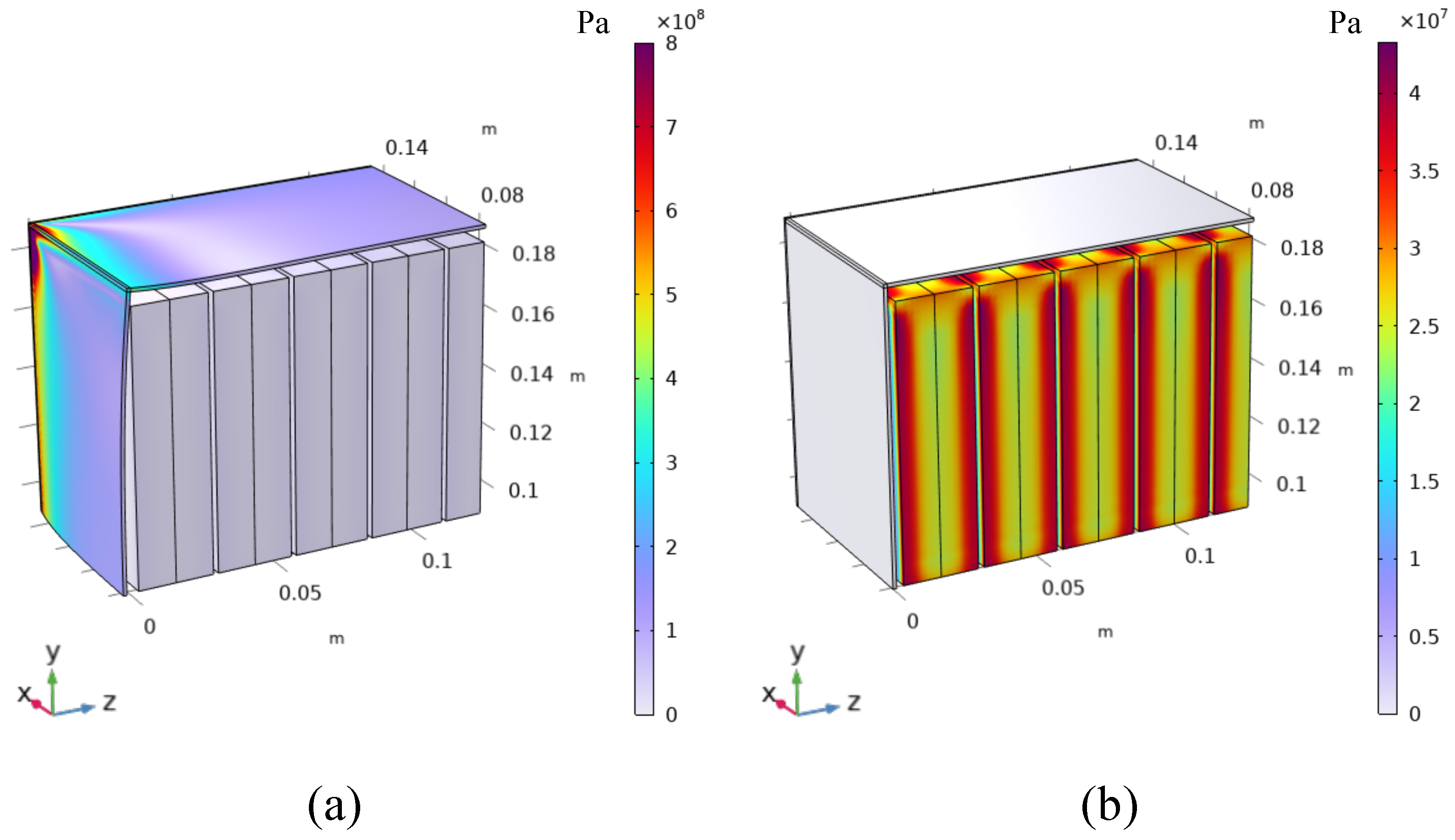
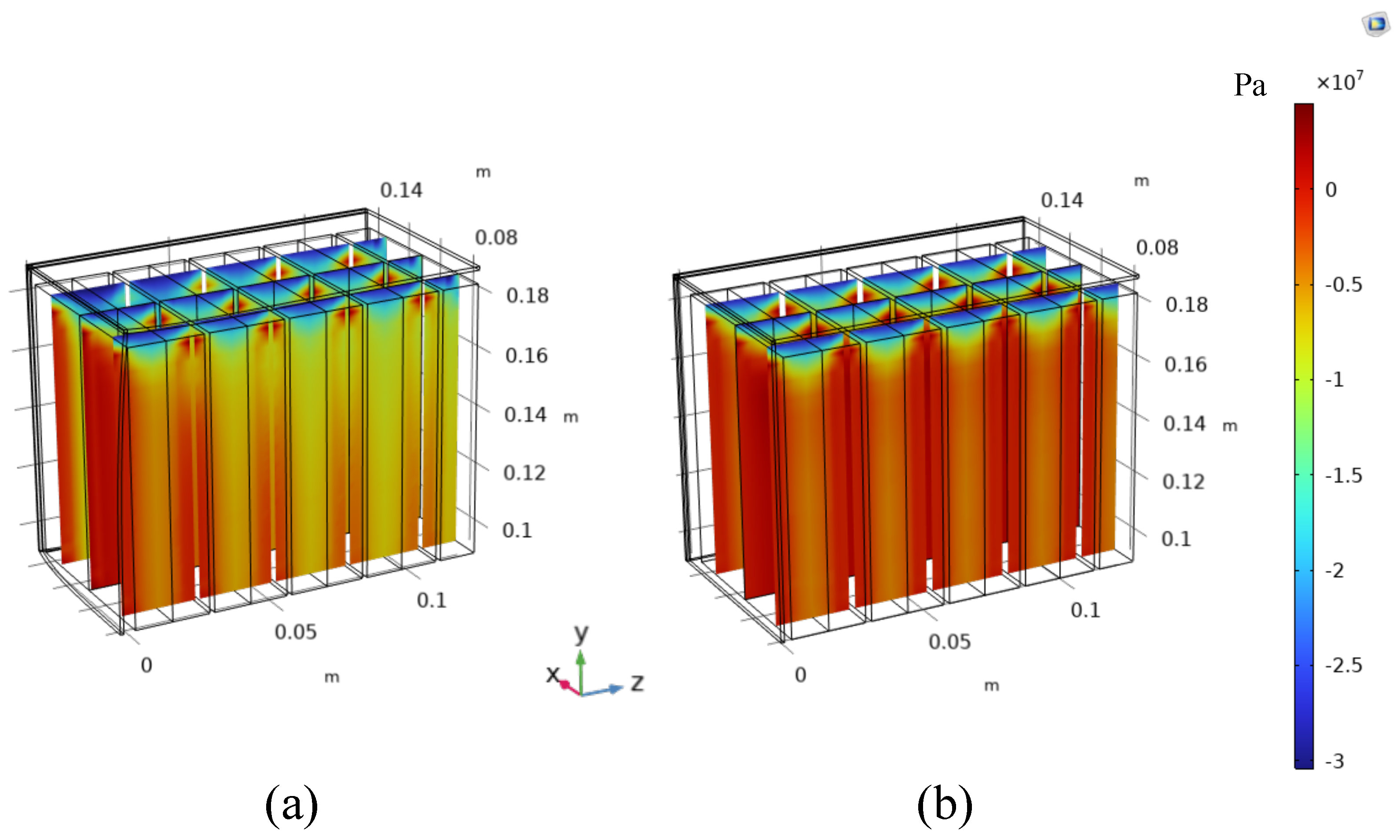
4.2. Battery Module Level
- Batteries are packed with no gaps between each other.
- A gap equal to 0.5 mm is left between the batteries. This gap could be also smaller, at most equal to the half of the reversible deformation computed or measured for each single battery (0.2 mm in this case). Nevertheless, from a constructive point of view, it would be difficult to leave such a small gap. For this reason, 0.5 mm is chosen as a realistic and conservative scenario.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martelli, S.; Mocera, F.; Somà, A. Carbon footprint of an orchard tractor through a life-cycle assessment approach. Agriculture 2023, 13, 1210. [Google Scholar] [CrossRef]
- Martelli, S.; Martini, V.; Mocera, F.; Soma’, A. Life Cycle Assessment Comparison of Orchard Tractors Powered by Diesel and Hydrogen Fuel Cell. Energies 2024, 17, 4599. [Google Scholar] [CrossRef]
- Martelli, S.; Mocera, F.; Soma, A. Autonomous Driving Strategy for a Specialized Four-Wheel Differential-Drive Agricultural Rover. AgriEngineering 2024, 6, 1937–1958. [Google Scholar] [CrossRef]
- Clerici, D. Mechanics of Lithium-Ion Batteries—A Modelling and Experimental Perspective. Ph.D. Thesis, Department of Mechanical and Aerospace Engineering, Politecnico di Torino, Turin, Italy, 2024. [Google Scholar]
- Clerici, D.; Pistorio, F.; Mocera, F.; Somà, A. Mechanical characterization and modelling of lithium-ion batteries. Transp. Res. Procedia 2023, 70, 276–283. [Google Scholar]
- Clerici, D.; Mocera, F.; Somà, A. Analytical solution for coupled diffusion induced stress model for lithium-ion battery. Energies 2020, 13, 1717. [Google Scholar] [CrossRef]
- Clerici, D.; Mocera, F.; Somà, A. Shape Influence of Active Material Micro-Structure on Diffusion and Contact Stress in Lithium-Ion Batteries. Energies 2020, 14, 134. [Google Scholar] [CrossRef]
- Clerici, D.; Mocera, F. Micro-scale modeling of Lithium-ion battery. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1038, 012007. [Google Scholar] [CrossRef]
- Clerici, D. Diffusion-induced stress amplification in phase-transition materials for electrodes of lithium-ion batteries. Int. J. Mech. Sci. 2024, 281, 109541. [Google Scholar]
- Pistorio, F.; Clerici, D.; Mocera, F.; Somà, A. Review on the Experimental Characterization of Fracture in Active Material for Lithium-Ion Batteries. Energies 2022, 15, 9168. [Google Scholar] [CrossRef]
- Pistorio, F.; Clerici, D.; Mocera, F.; Somà, A. Review on the numerical modeling of fracture in active materials for lithium ion batteries. J. Power Sources 2023, 566, 232875. [Google Scholar]
- Pistorio, F.; Clerici, D.; Mocera, F.; Somà, A. Coupled electrochemical–mechanical model for fracture analysis in active materials of lithium ion batteries. J. Power Sources 2023, 580, 233378. [Google Scholar]
- Clerici, D.; Pistorio, F.; Somà, A. Design and fracture mechanics of lithium-ion batteries. Procedia Struct. Integr. 2024, 58, 23–29. [Google Scholar] [CrossRef]
- Pistorio, F.; Clerici, D.; Somà, A. Analytical computation of stress intensity factor for active material particles of lithium ion batteries. Eng. Fract. Mech. 2023, 292, 109597. [Google Scholar]
- Pistorio, F.; Clerici, D. Analytical computation of stress intensity factor for multi-physics problems. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2024; Volume 1306, p. 012009. [Google Scholar]
- Clerici, D.; Mocera, F.; Pistorio, F. Analysis of fracture behaviour in active materials for lithium ion batteries. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2022; Volume 1214, p. 012018. [Google Scholar]
- Clerici, D.; Pistorio, F.; Somà, A. Aging diagnostics in lithium-ion batteries with differential mechanical measurements. Appl. Energy 2025, 386, 125524. [Google Scholar] [CrossRef]
- Birkl, C.R.; Roberts, M.R.; McTurk, E.; Bruce, P.G.; Howey, D.A. Degradation diagnostics for lithium ion cells. J. Power Sources 2017, 341, 373–386. [Google Scholar] [CrossRef]
- Gantenbein, S.; Schönleber, M.; Weiss, M.; Ivers-Tiffée, E. Capacity fade in lithium-ion batteries and cyclic aging over various state-of-charge ranges. Sustainability 2019, 11, 6697. [Google Scholar] [CrossRef]
- Chen, D.; Kramer, D.; Mönig, R. Chemomechanical fatigue of LiMn1.95Al0.05O4 electrodes for lithium-ion batteries. Electrochim. Acta 2018, 259, 939–948. [Google Scholar] [CrossRef]
- Mocera, F.; Somà, A.; Clerici, D. Study of aging mechanisms in lithium-ion batteries for working vehicle applications. In Proceedings of the 2020 Fifteenth International Conference on Ecological Vehicles and Renewable Energies (EVER), Monte-Carlo, Monaco, 10–12 September 2020; pp. 1–8. [Google Scholar]
- Clerici, D.; Mocera, F. Experimental Characterization of Lithium-Ion Cell Strain Using Laser Sensors. Energies 2021, 14, 6281. [Google Scholar] [CrossRef]
- Clerici, D.; Mocera, F.; Soma, A. Electrochemical–mechanical multi-scale model and validation with thickness change measurements in prismatic lithium-ion batteries. J. Power Sources 2022, 542, 231735. [Google Scholar]
- Mohtat, P.; Lee, S.; Sulzer, V.; Siegel, J.B.; Stefanopoulou, A.G. Differential Expansion and Voltage Model for Li-ion Batteries at Practical Charging Rates. J. Electrochem. Soc. 2020, 167, 110561. [Google Scholar] [CrossRef]
- Rieger, B.; Schlueter, S.; Erhard, S.V.; Schmalz, J.; Reinhart, G.; Jossen, A. Multi-scale investigation of thickness changes in a commercial pouch type lithium-ion battery. J. Energy Storage 2016, 6, 213–221. [Google Scholar] [CrossRef]
- Mohan, S.; Kim, Y.; Siegel, J.B.; Samad, N.A.; Stefanopoulou, A.G. A phenomenological model of bulk force in a li-ion battery pack and its application to state of charge estimation. J. Electrochem. Soc. 2014, 161, A2222. [Google Scholar] [CrossRef]
- Figueroa-Santos, M.A.; Siegel, J.B.; Stefanopoulou, A.G. Leveraging Cell Expansion Sensing in State of Charge Estimation: Practical Considerations. Energies 2020, 13, 2653. [Google Scholar] [CrossRef]
- Raghavan, A.; Kiesel, P.; Sommer, L.W.; Schwartz, J.; Lochbaum, A.; Hegyi, A.; Schuh, A.; Arakaki, K.; Saha, B.; Ganguli, A.; et al. Embedded fiber-optic sensing for accurate internal monitoring of cell state in advanced battery management systems part 1: Cell embedding method and performance. J. Power Sources 2017, 341, 466–473. [Google Scholar] [CrossRef]
- Ganguli, A.; Saha, B.; Raghavan, A.; Kiesel, P.; Arakaki, K.; Schuh, A.; Schwartz, J.; Hegyi, A.; Sommer, L.W.; Lochbaum, A.; et al. Embedded fiber-optic sensing for accurate internal monitoring of cell state in advanced battery management systems part 2: Internal cell signals and utility for state estimation. J. Power Sources 2017, 341, 474–482. [Google Scholar] [CrossRef]
- Nascimento, M.; Novais, S.; Ding, M.S.; Ferreira, M.S.; Koch, S.; Passerini, S.; Pinto, J.L. Internal strain and temperature discrimination with optical fiber hybrid sensors in Li-ion batteries. J. Power Sources 2019, 410, 1–9. [Google Scholar] [CrossRef]
- Willenberg, L.K.; Dechent, P.; Fuchs, G.; Sauer, D.U.; Figgemeier, E. High-precision monitoring of volume change of commercial lithium-ion batteries by using strain gauges. Sustainability 2020, 12, 557. [Google Scholar] [CrossRef]
- Hemmerling, J.; Fill, A.; Birke, K.P. Analysis of the age-, current-and temperature-dependent expansion of cylindrical NCM| Graphite Li-ion battery cells using strain gauges. J. Energy Storage 2024, 99, 113177. [Google Scholar]
- Jones, E.M.; Silberstein, M.N.; White, S.R.; Sottos, N.R. In Situ Measurements of Strains in Composite Battery Electrodes during Electrochemical Cycling. Exp. Mech. 2014, 54, 971–985. [Google Scholar] [CrossRef]
- Gold, L.; Bach, T.; Virsik, W.; Schmitt, A.; Müller, J.; Staab, T.E.; Sextl, G. Probing lithium-ion batteries’ state-of-charge using ultrasonic transmission—Concept and laboratory testing. J. Power Sources 2017, 343, 536–544. [Google Scholar] [CrossRef]
- Schmitt, J.; Kraft, B.; Schmidt, J.P.; Meir, B.; Elian, K.; Ensling, D.; Keser, G.; Jossen, A. Measurement of gas pressure inside large-format prismatic lithium-ion cells during operation and cycle aging. J. Power Sources 2020, 478, 228661. [Google Scholar] [CrossRef]
- Hahn, S.; Theil, S.; Kroggel, J.; Birke, K.P. Pressure Prediction Modeling and Validation for Lithium-Ion Pouch Cells in Buffered Module Assemblies. J. Energy Storage 2021, 40, 102517. [Google Scholar]
- Plotnikov, Y.; Karp, J.; Knobloch, A.; Kapusta, C.; Lin, D. Eddy current sensor for in-situ monitoring of swelling of Li-ion prismatic cells. AIP Conf. Proc. 2015, 1650, 434–442. [Google Scholar] [CrossRef]
- Clerici, D.; Martelli, S.; Mocera, F.; Somà, A. Mechanical characterization of lithium-ion batteries with different chemistries and formats. J. Energy Storage 2024, 84, 110899. [Google Scholar]
- Grimsmann, F.; Brauchle, F.; Gerbert, T.; Gruhle, A.; Knipper, M.; Parisi, J. Hysteresis and current dependence of the thickness change of lithium-ion cells with graphite anode. J. Energy Storage 2017, 12, 132–137. [Google Scholar] [CrossRef]
- Oh, K.Y.; Siegel, J.B.; Secondo, L.; Kim, S.U.; Samad, N.A.; Qin, J.; Anderson, D.; Garikipati, K.; Knobloch, A.; Epureanu, B.I.; et al. Rate dependence of swelling in lithium-ion cells. J. Power Sources 2014, 267, 197–202. [Google Scholar] [CrossRef]
- Sauerteig, D.; Hanselmann, N.; Arzberger, A.; Reinshagen, H.; Ivanov, S.; Bund, A. Electrochemical-mechanical coupled modeling and parameterization of swelling and ionic transport in lithium-ion batteries. J. Power Sources 2018, 378, 235–247. [Google Scholar]
- Gupta, P.; Gudmundson, P. A multi-scale model for simulation of electrochemically induced stresses on scales of active particles, electrode layers, and battery level in lithium-ion batteries. J. Power Sources 2021, 511, 230465. [Google Scholar]
- Gupta, P.; Gudmundson, P. Modeling of local electrode stresses and pressures in lithium-ion battery packs using three-dimensional homogenization. J. Power Sources 2023, 582, 233514. [Google Scholar] [CrossRef]
- Voigt, W. Ueber die Beziehung zwischen den beiden Elasticitätsconstanten isotroper Körper. Ann. Der Phys. 1889, 274, 573–587. [Google Scholar] [CrossRef]
- Reuß, A. Berechnung der Fließgrenze von Mischkristallen auf Grund der Plastizitätsbedingung für Einkristalle. Zamm-Z. Fur Angew. Math. Und Mech. 1929, 9, 49–58. [Google Scholar]
- Halpin, J.C. Effects of Environmental Factors on Composite Materials. Technical Report. 1969. Available online: https://apps.dtic.mil/sti/tr/pdf/ADA306357.pdf (accessed on 20 May 2024).
- Drago, A.; Pindera, M.J. Micro-macromechanical analysis of heterogeneous materials: Macroscopically homogeneous vs periodic microstructures. Compos. Sci. Technol. 2007, 67, 1243–1263. [Google Scholar] [CrossRef]
- Qi, Y.; Hector, L.G.; James, C.; Kim, K.J. Lithium Concentration Dependent Elastic Properties of Battery Electrode Materials from First Principles Calculations. J. Electrochem. Soc. 2014, 161, F3010. [Google Scholar] [CrossRef]
- Christensen, J.; Newman, J. Stress Generation and Fracture in Lithium Insertion Materials. J. Solid State Electrochem. 2006, 10, 293–319. [Google Scholar] [CrossRef]
- Sahraei, E.; Bosco, E.; Dixon, B.; Lai, B. Microscale failure mechanisms leading to internal short circuit in Li-ion batteries under complex loading scenarios. J. Power Sources 2016, 319, 56–65. [Google Scholar] [CrossRef]
- Prussin, S. Generation and Distribution of Dislocations by Solute Diffusion. J. Appl. Phys. 1961, 32, 1876–1881. [Google Scholar] [CrossRef]
- Koerver, R.; Zhang, W.; de Biasi, L.; Schweidler, S.; Kondrakov, A.O.; Kolling, S.; Brezesinski, T.; Hartmann, P.; Zeier, W.G.; Janek, J. Chemo-mechanical expansion of lithium electrode materials–on the route to mechanically optimized all-solid-state batteries. Energy Environ. Sci. 2018, 11, 2142–2158. [Google Scholar]
- Doyle, M.; Fuller, T.F.; Newman, J. Modeling of galvanostatic charge and discharge of the lithium/polymer/insertion cell. J. Electrochem. Soc. 1993, 140, 1526. [Google Scholar]
- Newman, J.; Tiedemann, W. Porous-electrode theory with battery applications. AIChE J. 1975, 21, 25–41. [Google Scholar] [CrossRef]
- Dao, T.S.; Vyasarayani, C.P.; McPhee, J. Simplification and order reduction of lithium-ion battery model based on porous-electrode theory. J. Power Sources 2012, 198, 329–337. [Google Scholar] [CrossRef]
- Rieger, B.; Schlueter, S.; Erhard, S.V.; Jossen, A. Strain propagation in lithium-ion batteries from the crystal structure to the electrode level. J. Electrochem. Soc. 2016, 163, A1595. [Google Scholar]
- Taminato, S.; Yonemura, M.; Shiotani, S.; Kamiyama, T.; Torii, S.; Nagao, M.; Ishikawa, Y.; Mori, K.; Fukunaga, T.; Onodera, Y.; et al. Real-time observations of lithium battery reactions—operando neutron diffraction analysis during practical operation. Sci. Rep. 2016, 6, 1–12. [Google Scholar]

| Domain | Property | Symbol | Value | Unit |
|---|---|---|---|---|
| Cathode Active Layer | Particle Young’s Modulus | 125 l [48] | GPa | |
| Particle Poisson’s ratio | 0.3 l [48] | - | ||
| Particle radius | 0.03 m | m | ||
| Porosity | 0.608 c | - | ||
| Thickness | 72 m | m | ||
| Anode Active Layer | Particle Young’s Modulus | 15 l [49] | GPa | |
| Particle Poisson’s ratio | 0.3 l [49] | - | ||
| Particle radius | 10 m | m | ||
| Porosity | 0.434 c | - | ||
| Thickness | 60 m | m | ||
| Cathode Current Collector | Young’s Modulus | 70 l | GPa | |
| Poisson’s ratio | 0.3 l | - | ||
| Thickness | 12 m | m | ||
| Anode Current Collector | Young’s Modulus | 110 l | GPa | |
| Poisson’s ratio | 0.3 l | - | ||
| Thickness | 10 m | m | ||
| Separator | Young’s Modulus | 0.4 l [50] | GPa | |
| Poisson’s ratio | 0.01 l [50] | - | ||
| Thickness | 33 m | m |
| Domain | Property | Symbol | Value | Unit |
|---|---|---|---|---|
| Battery Case | Young’s Modulus | 70 | GPa | |
| Poisson’s ratio | 0.33 | - | ||
| Thickness | 0.8 | mm | ||
| Width | 70 | mm | ||
| Height | 27 | mm | ||
| Length | 185 | mm | ||
| Module Case | Young’s Modulus | 210 | GPa | |
| Poisson’s ratio | 0.3 | - | ||
| Thickness | 1.2 | mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clerici, D.; Pistorio, F.; Scalzo, S.; Martelli, S.; Mocera, F.; Somà, A. Mechanical Multiscale Lithium-Ion Battery Modeling for Optimized Battery Pack Design. Eng. Proc. 2025, 85, 48. https://doi.org/10.3390/engproc2025085048
Clerici D, Pistorio F, Scalzo S, Martelli S, Mocera F, Somà A. Mechanical Multiscale Lithium-Ion Battery Modeling for Optimized Battery Pack Design. Engineering Proceedings. 2025; 85(1):48. https://doi.org/10.3390/engproc2025085048
Chicago/Turabian StyleClerici, Davide, Francesca Pistorio, Salvatore Scalzo, Salvatore Martelli, Francesco Mocera, and Aurelio Somà. 2025. "Mechanical Multiscale Lithium-Ion Battery Modeling for Optimized Battery Pack Design" Engineering Proceedings 85, no. 1: 48. https://doi.org/10.3390/engproc2025085048
APA StyleClerici, D., Pistorio, F., Scalzo, S., Martelli, S., Mocera, F., & Somà, A. (2025). Mechanical Multiscale Lithium-Ion Battery Modeling for Optimized Battery Pack Design. Engineering Proceedings, 85(1), 48. https://doi.org/10.3390/engproc2025085048








