Utilization of Waste Graphite for the Sustainable Production of Silicon Carbide †
Abstract
1. Introduction
2. Theoretical Approach
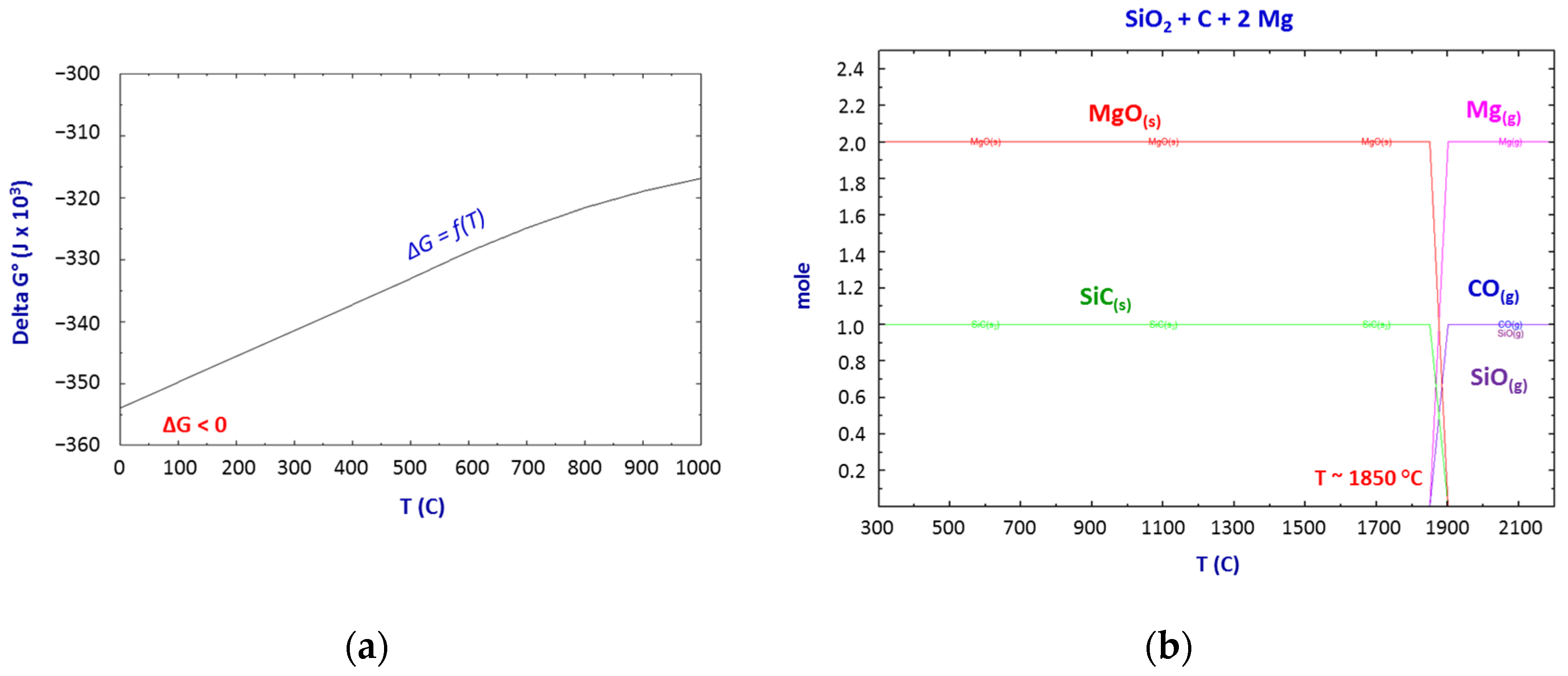
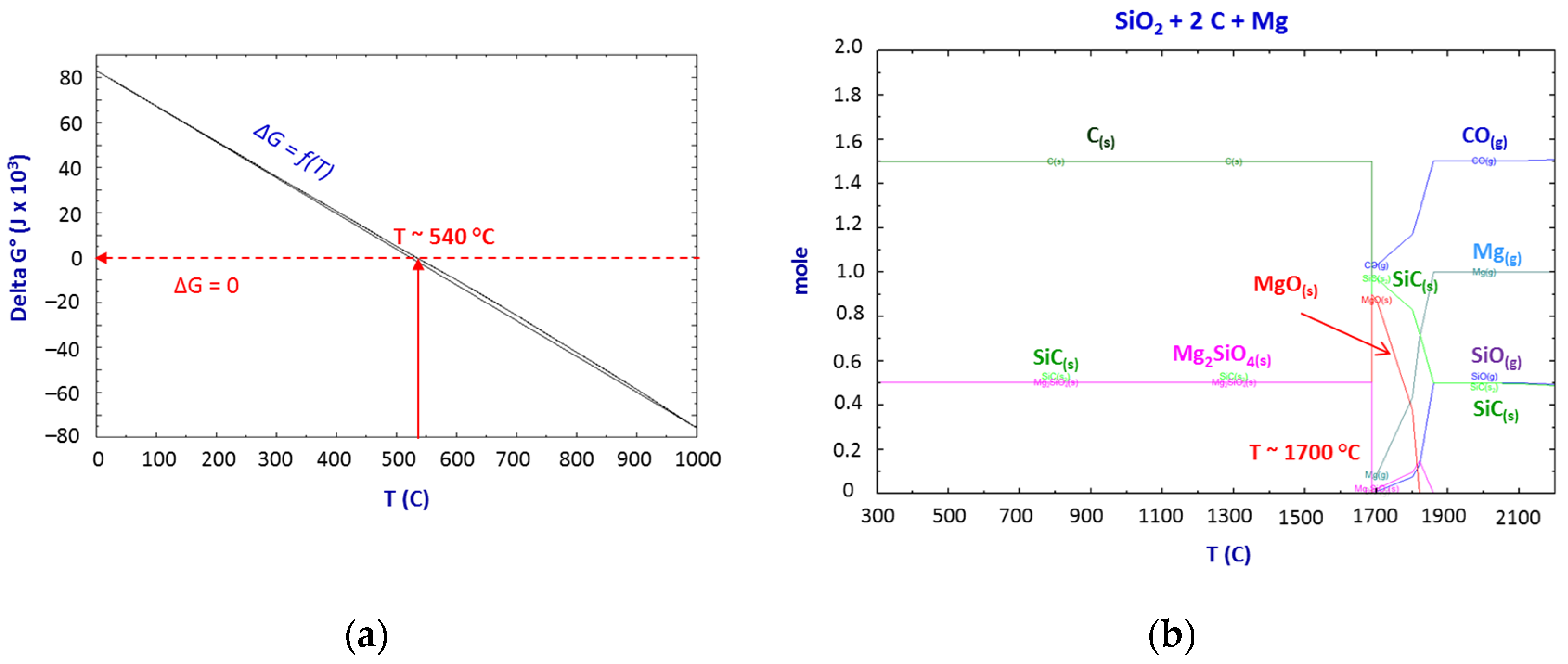
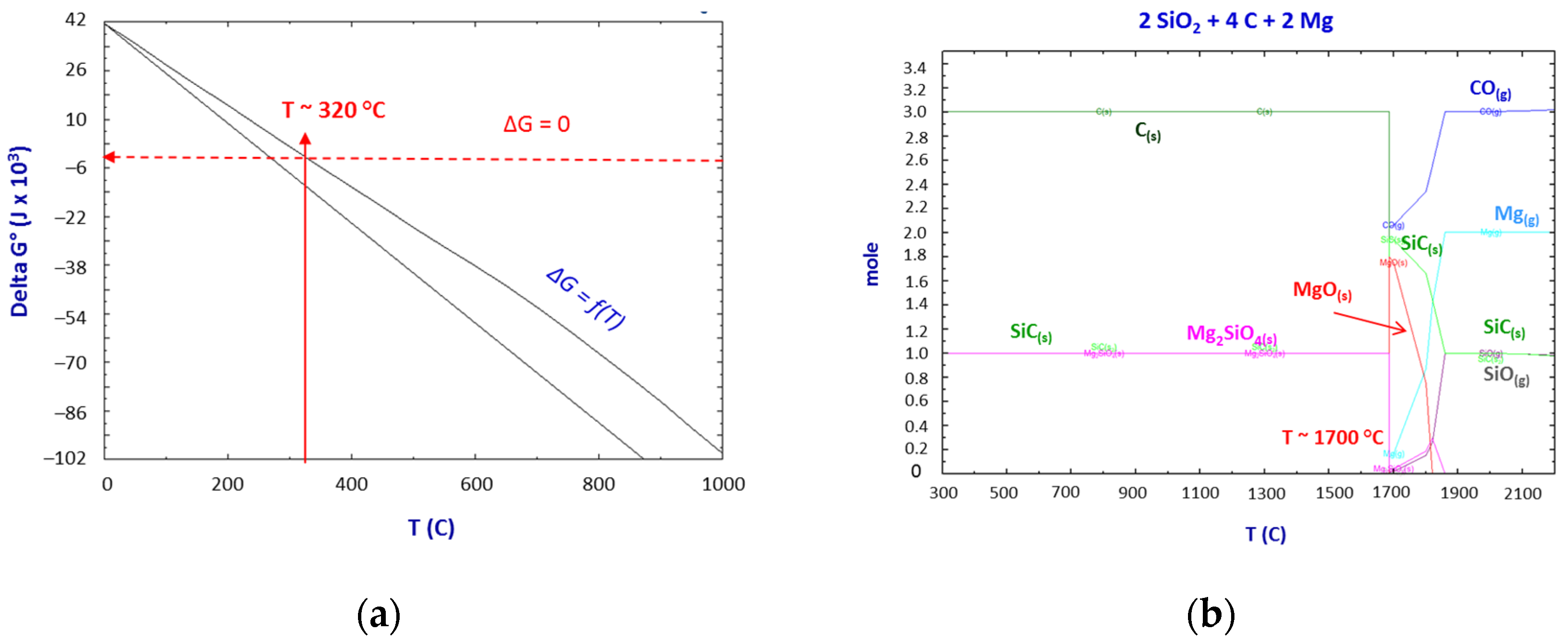
Thermodynamics of the SiO2–C–Mg System Reactions
3. Materials and Methods
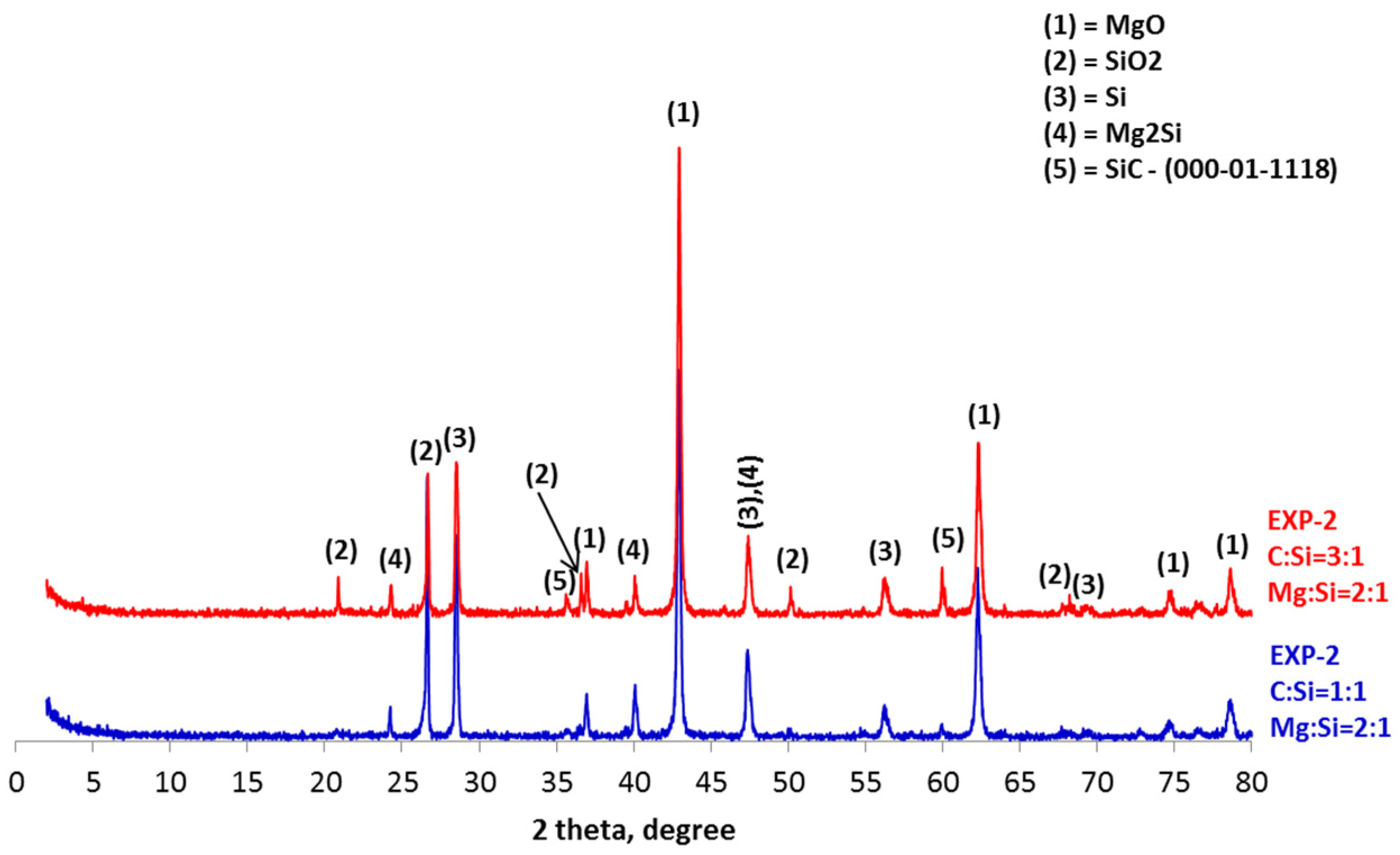
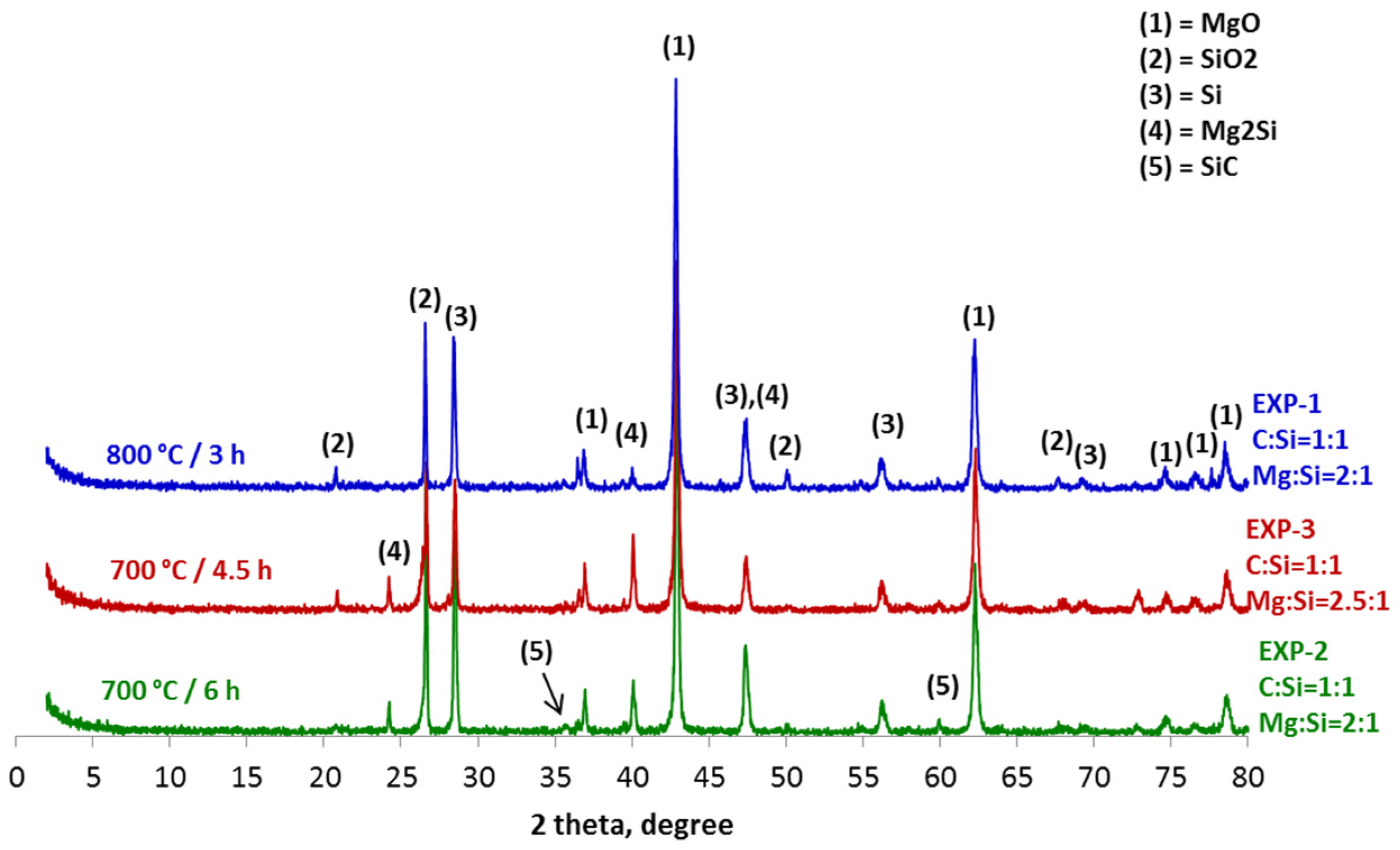
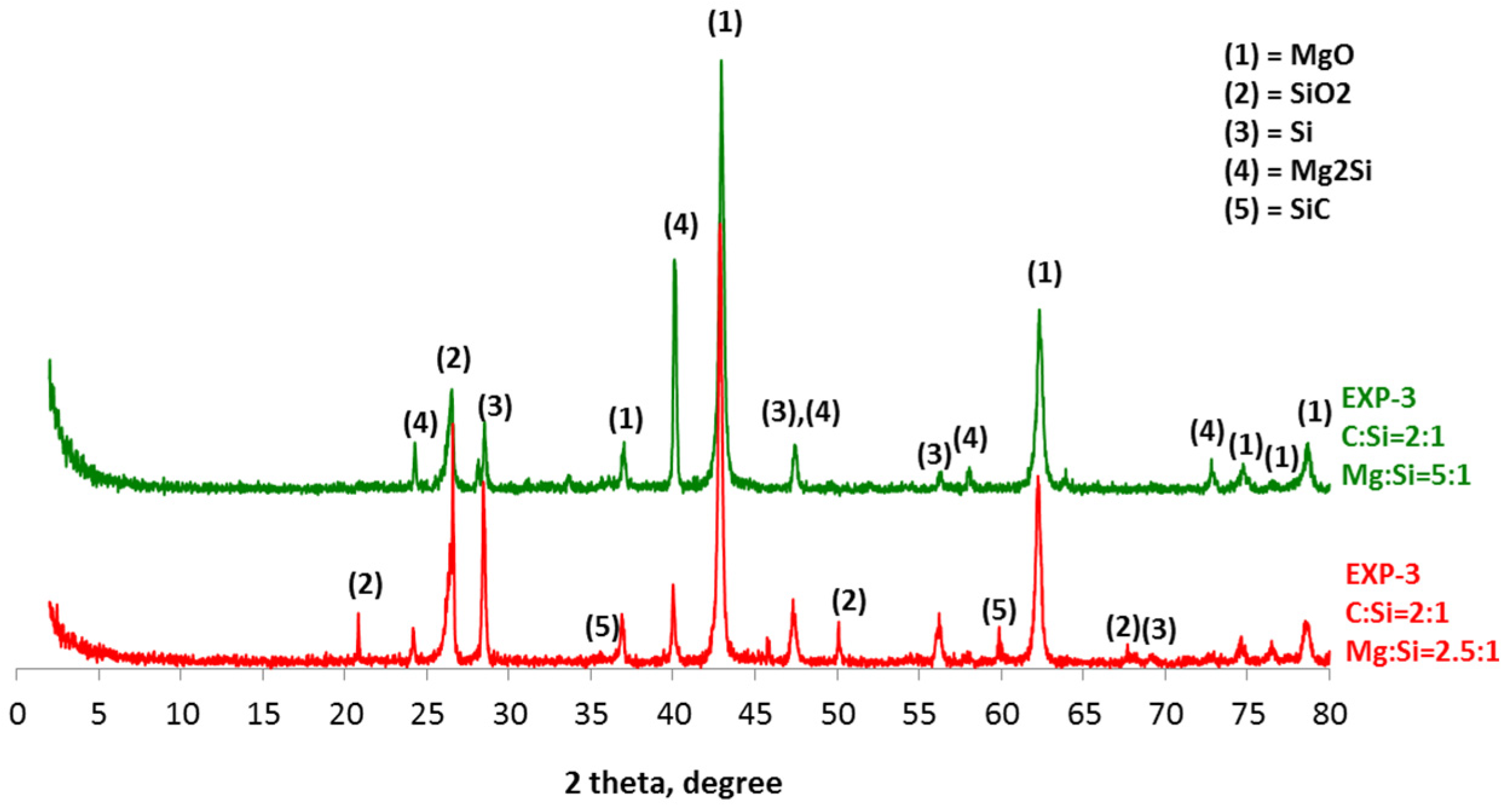
4. Results and Discussion
5. Conclusions
- The sustainable production of silicon carbide by utilizing the waste graphite generated in industrial processes is feasible through a magnesiothermal reduction process that takes place at temperatures below 1000 °C.
- The thermodynamic study of the system SiO2-C-Mg confirms the potential of SiC formation at temperatures below 1000 °C.
- The molar ratios of SiO2 to C and SiO2 to Mg proved to be significant parameters of the process.
- The synthesis of SiC following the magnesiothermal reduction of silicon oxide proceeds via intermediate reaction steps and products, which define the rate of the overall reaction.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fend, Z.C. SiC Power Materials: Devices and Applications; Springer Series in Material Science; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Mohd Sohor, M.A.H.; Mustapha, M.; Kurnia, J.C. Silicon carbide- from synthesis to application: A review. MATEC Web Conf. 2017, 131, 04003. [Google Scholar] [CrossRef]
- Thulasiraman, A.V.; Ganesapillai, M. A Systematic Review on the Synthesis of Silicon Carbide: An Alternative Approach to Valorisation of Residual Municipal Solid Waste. Processes 2023, 11, 283. [Google Scholar] [CrossRef]
- Abderrazak, H.; Hadj Hmi, E.S.B. Silicon Carbide: Synthesis and Properties. In Properties and Applications of Silicon Carbide; Gerhardt, R., Ed.; InTech: London, UK, 2011. [Google Scholar] [CrossRef]
- Li, X.; Zhang, G.; Tang, K.; Ostrovski, O.; Tronstad, R. Synthesis of silicon carbide by carbothermal reduction of quartz in H2-Ar gas mixtures. In Proceedings of the Fourteenth International Ferroalloys Congress “Energy Efficiency and Environmental Friendliness Are the Future of the Global Ferroalloy Industry”, Kiev, Ukraine, 31 May–4 June 2015. [Google Scholar]
- Gubernat, A.; Pichor, W.; Lach, R.; Zientara, D.; Sitarz, M.; Springwald, M. Low-temperature synthesis of silicon carbide powder using shungite. Boletín Soc. Española Cerámica Vidr. 2017, 56, 39–46. [Google Scholar] [CrossRef]
- Hidayat, N.; Ardiansyah, A.; Abdulloh, F.; Mufti, N.; Fibriyanti, A.A.; Yogihati, C.Y.; Prihandoko, B. Magnesiothermic reduction synthesis of silicon carbide with varying temperatures: Structural and mechanical features. Conf. Ser. Mater. Sci. Eng. 2019, 515, 012079. [Google Scholar] [CrossRef]
- Dasog, M.; Smith, F.L.; Purkait, T.; Veinot, J. Low temperature synthesis of silicon carbide nanomaterials using a solid-state method. Chem. Commun. 2013, 49, 7004. [Google Scholar] [CrossRef] [PubMed]
- Fuad, A.; Kultsum, U.; Taufiq, A.; Hartatiek, H. Low-temperature synthesis of α-SiC (6H-SiC) nanoparticles with magnesium catalyst. Mater. Today Proc. 2019, 17, 1451–1457. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, H.; Tao, H.; Tan, Z.; Jiao, Z.; Wu, M. Low temperature synthesis of mesoporous silicon carbide via magnesiothermic reduction. Mater. Lett. 2011, 65, 1552–1555. [Google Scholar] [CrossRef]
- Maroufi, S.; Mayyas, M.; Sahajwalla, V. Waste materials conversion into mesoporous silicon carbide nanocermics: Nanofibre/particle mixture. J. Clean. Prod. 2017, 157, 213–221. [Google Scholar] [CrossRef]
- Yang, J.; Feng, J.; Li, W.; Chen, X.; Liu, X.; Ruan, J.; Qiu, R.; Xiong, Y.; Tian, S. A resource-utilization way of the waste printed circuit boards to prepare silicon carbide nanoparticles and their photocatalytic application. J. Hazard. Mater. 2019, 373, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Zhang, L.; Yuan, J.; Yao, Z.; Tang, L.; Wang, Z.; Zhang, Z. Co-utilization of spent pot-lining and coal gangue by hydrothermal acid-leaching method to prepare silicon carbide powder. J. Clean. Prod. 2018, 204, 848–860. [Google Scholar] [CrossRef]
- Hossain, S.T.; Johra, F.T.; Jung, W.-G. Fabrication of Silicon Carbide from Recycled Silicon Wafer Cutting Sludge and Its Purification. Appl. Sci. 2018, 8, 1841. [Google Scholar] [CrossRef]
- Riikonen, J.; Rantanen, J.; Thapa, R.; Le, N.T.; Rigolet, S.; Fioux, P.; Turhanen, P.; Bodiford, N.K.; Kalluri, J.R.; Ikonen, T.; et al. Rapid synthesis of nanostructured porous silicon carbide from biogenic silica. J. Am. Ceram. Soc. 2021, 104, 766–775. [Google Scholar] [CrossRef]
- Tan, C.; Liu, J.; Zhang, H.; Wang, J.; Li, S.; Song, J.; Zhang, Y.; Zhang, S. Low temperature synthesis of 2H-SiC powders via molten-salt-mediated magnesiothermic reduction. Ceram. Int. 2017, 43, 2431–2437. [Google Scholar] [CrossRef]
| Tests Series | T (°C) | t (h) | Molar Ratios | |
|---|---|---|---|---|
| C:SiO2 | Mg:SiO2 | |||
| EXP 1 | 800 | 3 | 1:1 | 1:1 |
| 2:1 | ||||
| 2:1 | 1:1 | |||
| 2:1 | ||||
| EXP 2 | 700 | 6 | 1:1 | 2:1 |
| 2:1 | ||||
| 3:1 | ||||
| EXP 3 | 700 | 4.5 | 1:1 | 2.5:1 |
| 5:1 | ||||
| 2:1 | 2.5:1 | |||
| 5:1 | ||||
| 3:1 | 2.5:1 | |||
| 5:1 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vourgidi, C.; Giannopoulou, I.; Kourtis, A.; Magganiari, M.; Xenidis, A. Utilization of Waste Graphite for the Sustainable Production of Silicon Carbide. Mater. Proc. 2023, 15, 82. https://doi.org/10.3390/materproc2023015082
Vourgidi C, Giannopoulou I, Kourtis A, Magganiari M, Xenidis A. Utilization of Waste Graphite for the Sustainable Production of Silicon Carbide. Materials Proceedings. 2023; 15(1):82. https://doi.org/10.3390/materproc2023015082
Chicago/Turabian StyleVourgidi, Charikleia, Ioanna Giannopoulou, Apostolos Kourtis, Maria Magganiari, and Anthimos Xenidis. 2023. "Utilization of Waste Graphite for the Sustainable Production of Silicon Carbide" Materials Proceedings 15, no. 1: 82. https://doi.org/10.3390/materproc2023015082
APA StyleVourgidi, C., Giannopoulou, I., Kourtis, A., Magganiari, M., & Xenidis, A. (2023). Utilization of Waste Graphite for the Sustainable Production of Silicon Carbide. Materials Proceedings, 15(1), 82. https://doi.org/10.3390/materproc2023015082







