Green Innovation: Harnessing Chitosan Hydrogel Beads for Sustainable Lead Removal in Wastewater Treatment towards Qatar Vision 2030 †
Abstract
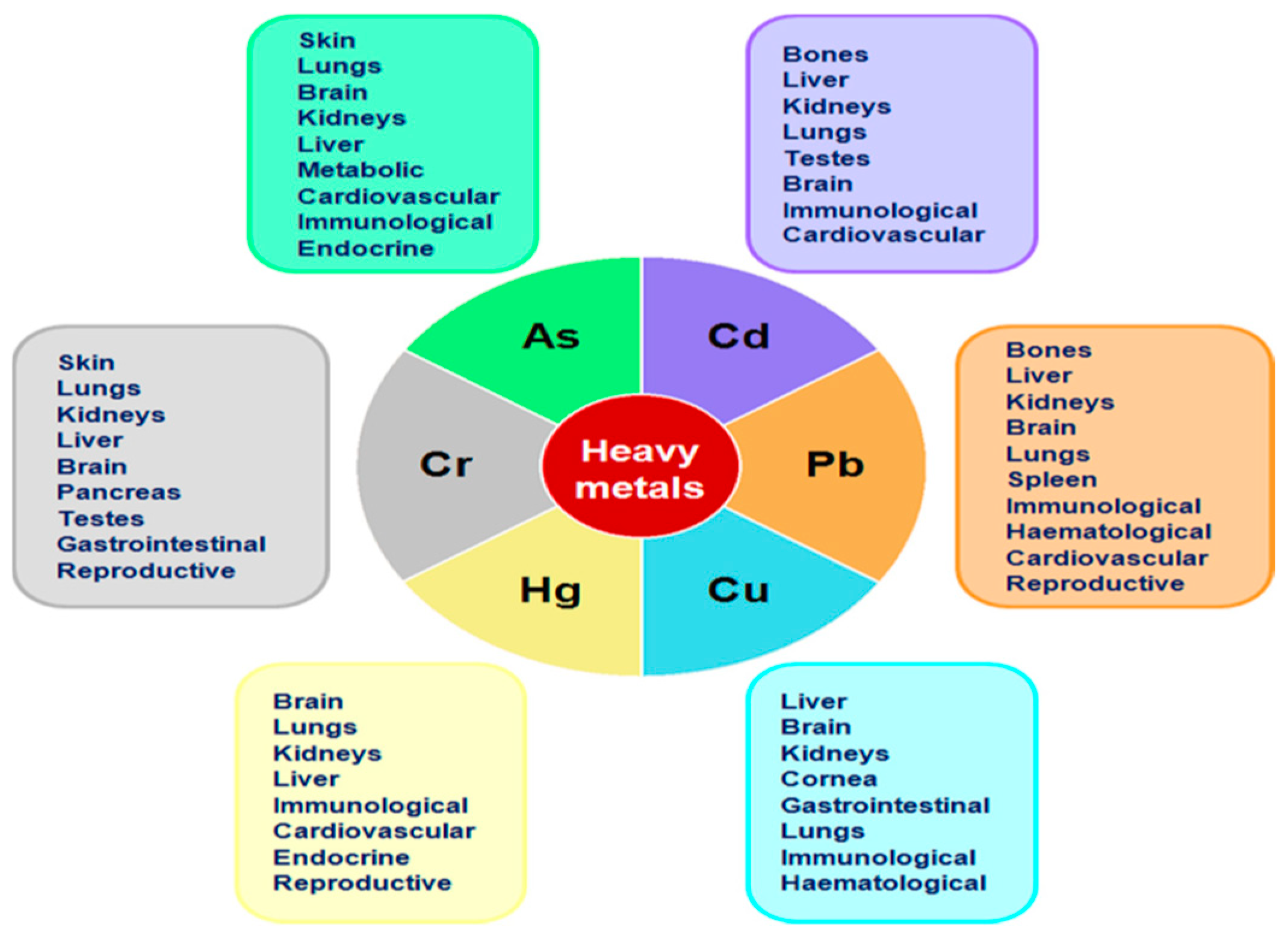
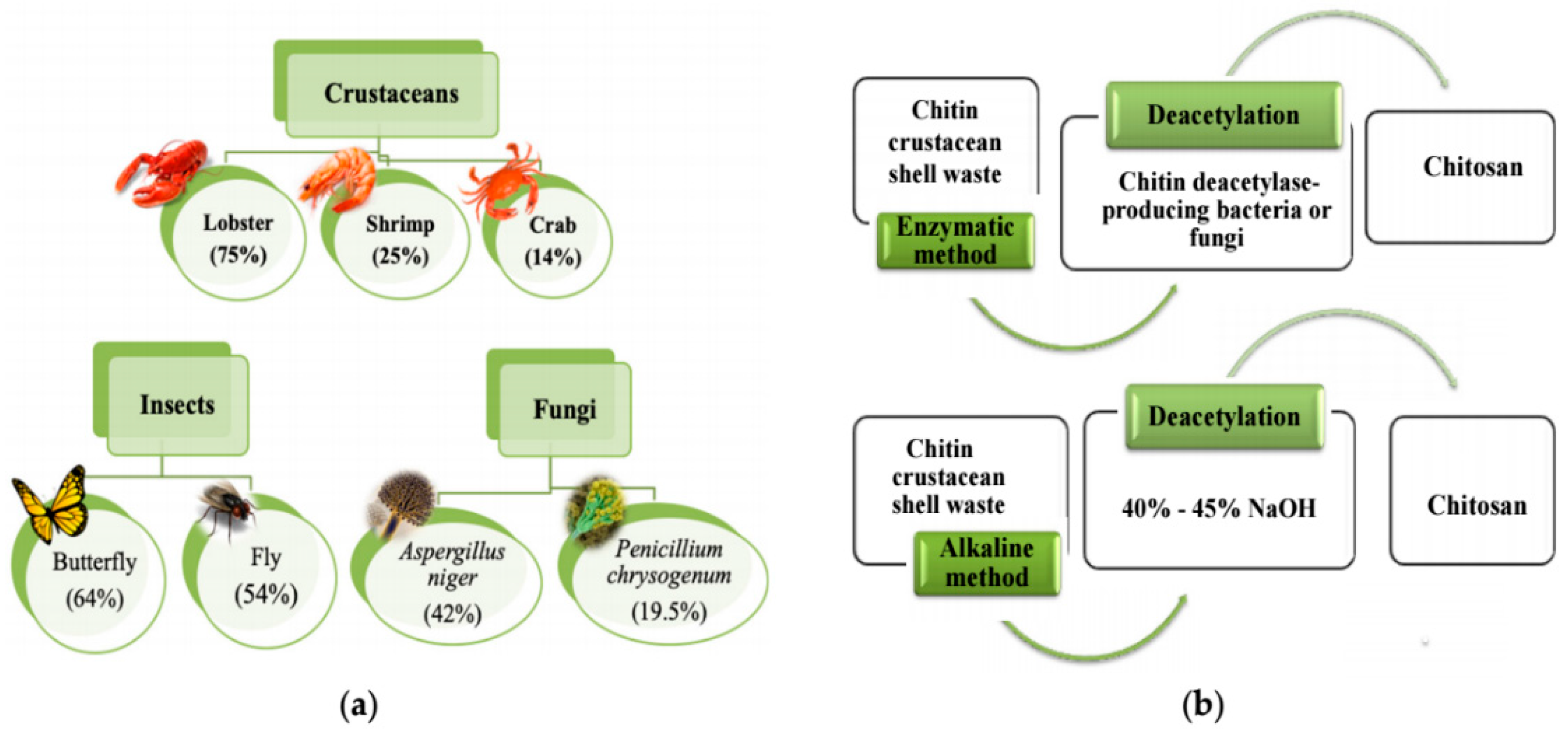
1. Introduction
2. Experiment
2.1. Procedure
2.2. Analysis
3. Results and Discussion
3.1. ICP-OES Results
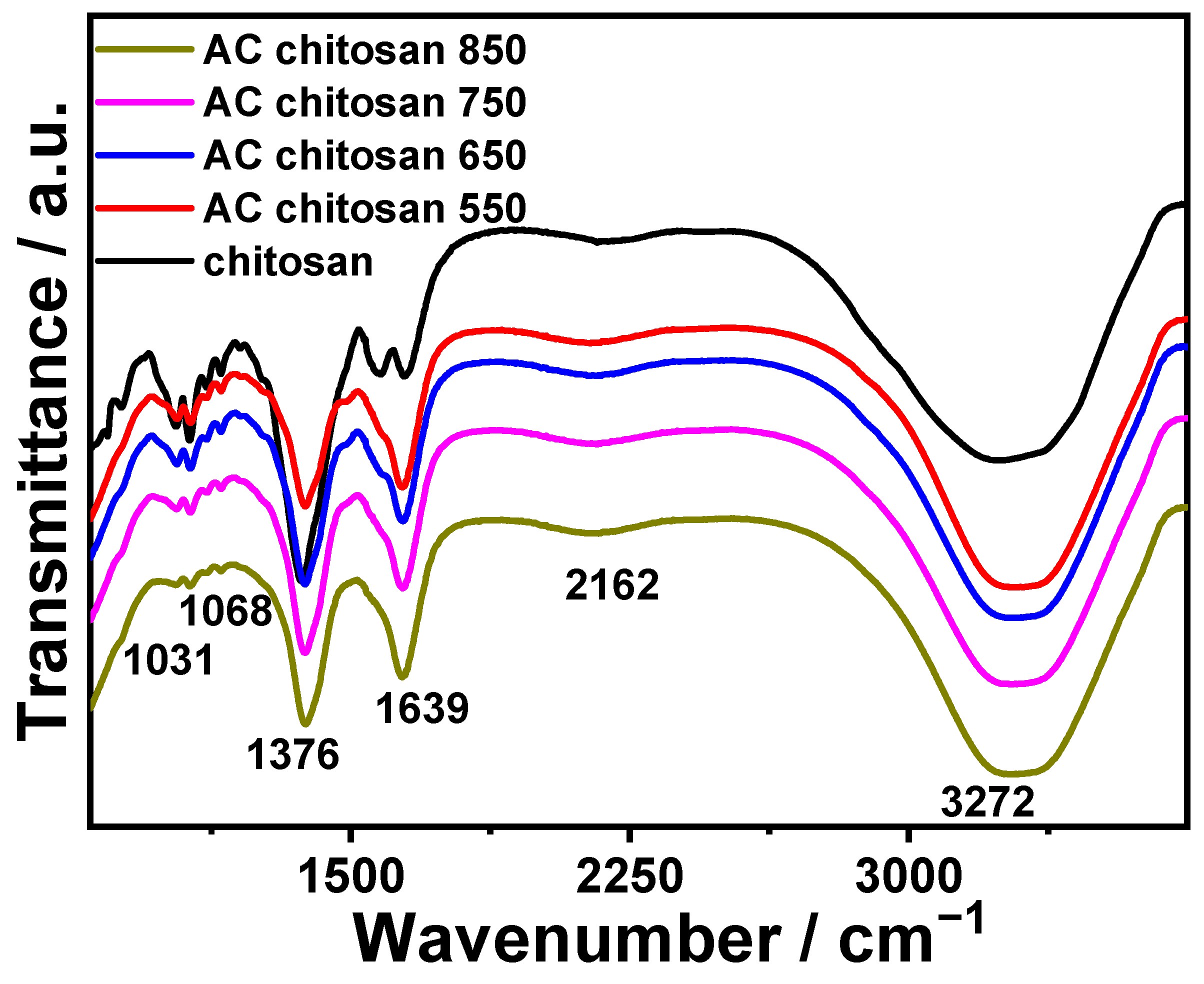
3.2. FTIR
3.3. SEM
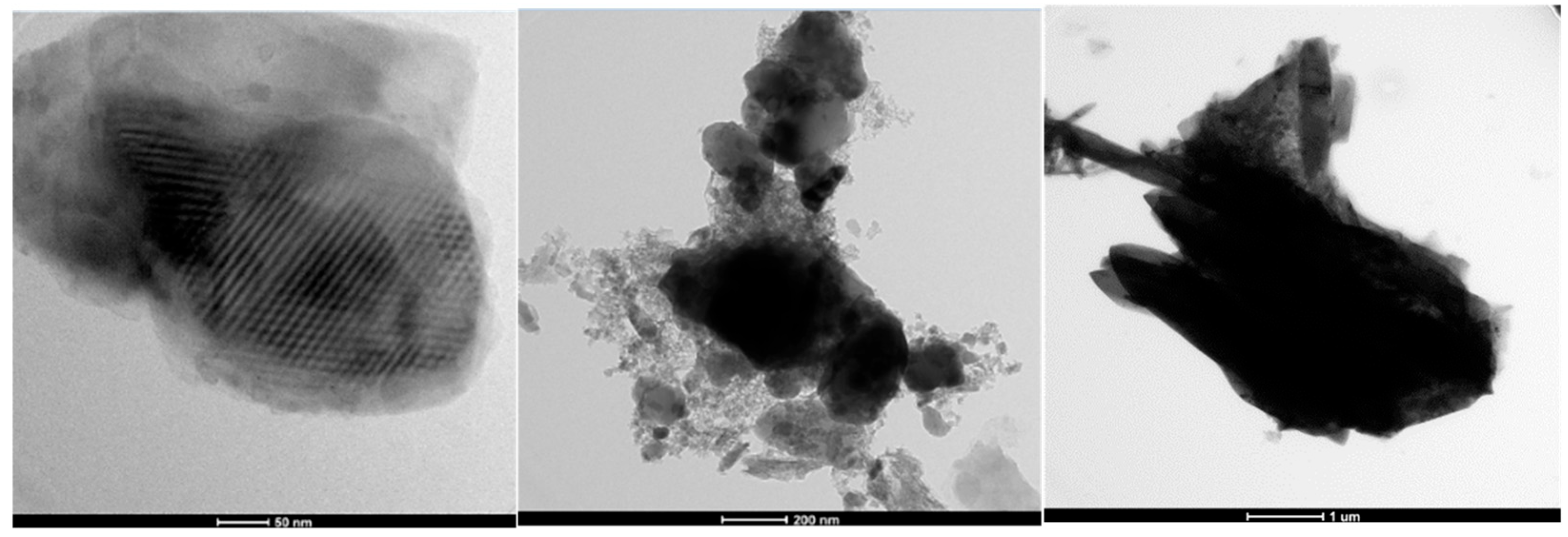
3.4. TEM
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, I.; Alharbi, O.M.; Tkachev, A.G.; Galunin, E.; Burakov, A.E.; Grachev, V.A. Water treatment by new-generation graphene materials: Hope for bright future. Environ. Sci. Pollut. Res. 2018, 25, 7315–7329. [Google Scholar] [CrossRef] [PubMed]
- Basheer, A.A. New generation nano-adsorbents for the removal of emerging contaminants in water. J. Mol. Liq. 2018, 261, 583–593. [Google Scholar] [CrossRef]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.Q. Heavy metals and pesticides toxicity in agricultural soil and plants: Ecological risks and human health implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.Z.; Salleh, W.N.; Ismail, A.F.; Yusof, N.; Yusop, M.Z.; Aziz, F. Adsorptive removal of heavy metal ions using graphene-based nanomaterials: Toxicity, roles of functional groups and mechanisms. Chemosphere 2020, 248, 126008. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef]
- Qasem, N.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. npj Clean Water 2021, 4, 1–5. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Cui, Y.; Chen, N. Removal of copper ions from wastewater: A review. Int. J. Environ. Res. Public Health 2023, 20, 3885. [Google Scholar] [CrossRef]
- El Batouti, M.; Al-Harby, N.F.; Elewa, M.M. A review on promising membrane technology approaches for heavy metal removal from water and wastewater to solve water crisis. Water 2021, 13, 3241. [Google Scholar] [CrossRef]
- Pal, K.; Sarkar, P.; Anis, A.; Wiszumirska, K.; Jarzębski, M. Polysaccharide-Based Nanocomposites for Food Packaging Applications. Materials 2021, 14, 5549. [Google Scholar] [CrossRef]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A natural biopolymer with a wide and varied range of applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Ajaj, Y.; Mahmoud, Z.H.; Ghadir, G.K.; Alani, Z.K.; Hussein, M.M.; Hussein, S.A.; Karim, M.M.; Al-khalidi, A.; Abbas, J.K.; et al. Adsorption of heavy metal ions use chitosan/graphene nanocomposites: A review study. Results Chem. 2024, 7, 101332. [Google Scholar] [CrossRef]
- Baalousha, H.M.; Ouda, O.K. Domestic water demand challenges in Qatar. Arab. J. Geosci. 2017, 10, 537. [Google Scholar] [CrossRef]
- He, Y.; Zhang, P.; Wang, L. Adsorption and Removal of Cr6+, Cu2+, Pb2+, and Zn2+ from Aqueous Solution by Magnetic Nano-Chitosan. Molecules 2023, 28, 2607. [Google Scholar] [CrossRef]
- Yadav, M.; Goswami, P.; Paritosh, K.; Kumar, M.; Pareek, N.; Vivekanand, V. Seafood waste: A source for preparation of commercially employable chitin/chitosan materials. Bioresour. Bioprocess. 2019, 6, 1–20. [Google Scholar] [CrossRef]
- da Silva Alves, D.C.; Healy, B.; Pinto, L.A.; Cadaval, T.R., Jr.; Breslin, C.B. Recent developments in chitosan-based adsorbents for the removal of pollutants from aqueous environments. Molecules 2021, 26, 594. [Google Scholar] [CrossRef]
- Harugade, A.; Sherje, A.P.; Pethe, A.M. Chitosan: A review on properties, biological activities and recent progress in biomedical applications. React. Funct. Polym. 2023, 191, 105634. [Google Scholar] [CrossRef]
- Kumar, N.; Chandan, N.K.; Bhushan, S.; Singh, D.K.; Kumar, S. Health risk assessment and metal contamination in fish, water and soil sediments in the East Kolkata Wetlands, India, Ramsar site. Sci. Rep. 2023, 13, 1546. [Google Scholar] [CrossRef]
- Pratiwi, R.; Prinajati, P.D. Adsorption for lead removal by chitosan from shrimp shells. Indones. J. Urban Environ. Technol. 2018, 2, 35–46. [Google Scholar] [CrossRef]
- Mohammad, A.M.; Eldin TA, S.; Hassan, M.A.; El-Anadouli, B.E. Efficient treatment of lead-containing wastewater by hydroxyapatite/chitosan nanostructures. Arab. J. Chem. 2017, 10, 683–690. [Google Scholar] [CrossRef]
- Zhang, C.J.; Hu, M.; Ke, Q.F.; Guo, C.X.; Guo, Y.J.; Guo, Y.P. Nacre-inspired hydroxyapatite/chitosan layered composites effectively remove lead ions in continuous-flow wastewater. J. Hazard. Mater. 2020, 386, 121999. [Google Scholar] [CrossRef]
- Karim, M.R.; Aijaz, M.O.; Alharth, N.H.; Alharbi, H.F.; Al-Mubaddel, F.S.; Awual, M.R. Composite nanofibers membranes of poly (vinyl alcohol)/chitosan for selective lead (II) and cadmium (II) ions removal from wastewater. Ecotoxicol. Environ. Saf. 2019, 169, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Sheth, Y.; Dharaskar, S.; Khalid, M.; Sonawane, S. An environment friendly approach for heavy metal removal from industrial wastewater using chitosan based biosorbent: A review. Sustain. Energy Technol. Assess. 2021, 43, 100951. [Google Scholar] [CrossRef]
- Rahman, A. Promising and Environmentally Friendly Removal of Copper, Zinc, Cadmium, and Lead from Wastewater Using Modified Shrimp-Based Chitosan. Water 2024, 16, 184. [Google Scholar] [CrossRef]
- Xu, H.; Li, X.; Gao, M.; Hu, X.; Zhang, X.; Li, Y.; Xu, X.; Hu, J.; Tang, C.; Hu, X. Chitosan and biochar synergize the efficient elimination of lead from wastewater by sulfidised nano-zero-valent iron. J. Environ. Chem. Eng. 2022, 10, 107101. [Google Scholar] [CrossRef]
- Frost, R.L.; López, A.; Theiss, F.L.; Aarão, G.M.; Scholz, R. A vibrational spectroscopic study of the phosphate mineral rimkorolgite (Mg, Mn2+) 5 (Ba, Sr)(PO4)4· 8H2O from Kovdor massif, Kola Peninsula, Russia. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 132, 762–766. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Anjeh, A.M.; Nabavi, S.R. Preparation, characterization and properties of a novel electrospun polyamide-6/chitosan/graphene oxide composite nanofiber. J. Polym. Environ. 2022, 30, 3934–3948. [Google Scholar] [CrossRef]
- Atangana, E.; Chiweshe, T.T.; Roberts, H. Modification of novel chitosan-starch cross-linked derivatives polymers: Synthesis and characterization. J. Polym. Environ. 2019, 27, 979–995. [Google Scholar] [CrossRef]

| Physicochemical properties. |
|
| Biological properties. |
|
| Sample (Pb) | Results Obtained (mg/L) |
|---|---|
| Lead initial concentration (100 ppm) | 109.554 |
| Chitosan | 3.859 |
| AC chitosan 550 | 11.489 |
| AC chitosan 650 | 21.587 |
| AC chitosan 750 | 12.723 |
| AC chitosan 850 | 12.485 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, G.; Helally, M.; Alani, M.A.F.; Alardah, A.H.S.; Khataby, R.A.M.; Fazili, M.Y.; Al-Maki, J.H.A.; Mohamed, A.; Sliem, M.H.R.; Al-Qahtani, N. Green Innovation: Harnessing Chitosan Hydrogel Beads for Sustainable Lead Removal in Wastewater Treatment towards Qatar Vision 2030. Mater. Proc. 2024, 18, 10. https://doi.org/10.3390/materproc2024018010
Ali G, Helally M, Alani MAF, Alardah AHS, Khataby RAM, Fazili MY, Al-Maki JHA, Mohamed A, Sliem MHR, Al-Qahtani N. Green Innovation: Harnessing Chitosan Hydrogel Beads for Sustainable Lead Removal in Wastewater Treatment towards Qatar Vision 2030. Materials Proceedings. 2024; 18(1):10. https://doi.org/10.3390/materproc2024018010
Chicago/Turabian StyleAli, Ghada, Mohamed Helally, Marwa A. F. Alani, Ala H. S. Alardah, Rinad A. M. Khataby, Maryam Y. Fazili, Jassim H. A. Al-Maki, Ali Mohamed, Mostafa H. R. Sliem, and Noora Al-Qahtani. 2024. "Green Innovation: Harnessing Chitosan Hydrogel Beads for Sustainable Lead Removal in Wastewater Treatment towards Qatar Vision 2030" Materials Proceedings 18, no. 1: 10. https://doi.org/10.3390/materproc2024018010
APA StyleAli, G., Helally, M., Alani, M. A. F., Alardah, A. H. S., Khataby, R. A. M., Fazili, M. Y., Al-Maki, J. H. A., Mohamed, A., Sliem, M. H. R., & Al-Qahtani, N. (2024). Green Innovation: Harnessing Chitosan Hydrogel Beads for Sustainable Lead Removal in Wastewater Treatment towards Qatar Vision 2030. Materials Proceedings, 18(1), 10. https://doi.org/10.3390/materproc2024018010







