3.1. Rheological Properties
The rheological properties of the coating suspensions are presented as the variation of shear viscosity as a function of shear rates over three subsequent cycles, as given in
Figure 1. The curves are recorded for suspensions with different fillers relative to the native natural rubber latex with solid content 60% (note: the viscosity scale of the materials is different for most detailed representations of the values). All filler types increased the viscosity of the original rubber latex to a different extent; however, all of them showed a shear-thinning effect with decreasing viscosity as a function of shear rate. The shear thinning behavior is enhanced in the presence of the fillers in most cases. The highest viscosities are observed for kaolinite fillers with an almost linear decrease in viscosity with shear rate at the highest concentrations, while the hysteresis of the kaolinite fillers is relatively low. This indicates the presence of strong mixing interactions between the kaolinite fillers and strong interactions with the natural rubber latex. The viscosity increase for SMI nanoparticles is significant with a more pronounced shear thinning effect, as the orientation of the nanoparticles under shear may additionally influence the structure of the suspension. The viscosity effects of nanoparticles are different from the microsize kaolinite, as an increase in nanoparticle concentration involves a decrease in viscosity. Therefore, it can be concluded from a viscosity-reducing effect of the nanoparticles that shear-induced mechanisms are influencing the nanoparticle mobility in the suspension and eventually lead to the orientation effects. The effects of reorganization of nanofillers in the rubber latex is also indicated by the relatively high hysteresis observed between the first and second ramp-up sequences for all concentrations, which was not observed for kaolinite fillers. Indeed, the nanoscale particles may have stronger influence on the latex flow properties compared to microscale particles. The latter are minimized in the case of talcum fillers, where very little alterations in viscosity and shear thinning effects are observed compared to the native rubber latex. However, the hysteresis effects for talc are also more pronounced than for kaolinite as it may be expected that the talc has a platelet structure that can be affected more by orientation effects under flow, while the kaolinite particles have a rather symmetrical shape. As it is observed that the viscosity for intermediate talc concentrations of 10 wt.-% decreases and the viscosity for the high talc concentrations of 20 wt.-% increases, the possible benefits of the orientation of the platelet structures are optimized at intermediate concentrations and hindered at the highest concentrations, where the highest concentrations might eventually lead to platelet–platelet interactions rather than platelet–latex interactions. The chemical interactions between the fillers and the natural rubber latex were not further studied at this stage, but besides particle shape, they can be attributed to specific surface interactions owing to the functional groups at the surface of the fillers, size distribution of the fillers and/or variations in zeta potential. While the present aim is to provide a view on the influence of the rheological properties on the coating formation, the latter interactions are the subject of more fundamental study in future.
3.2. Microstructural Properties
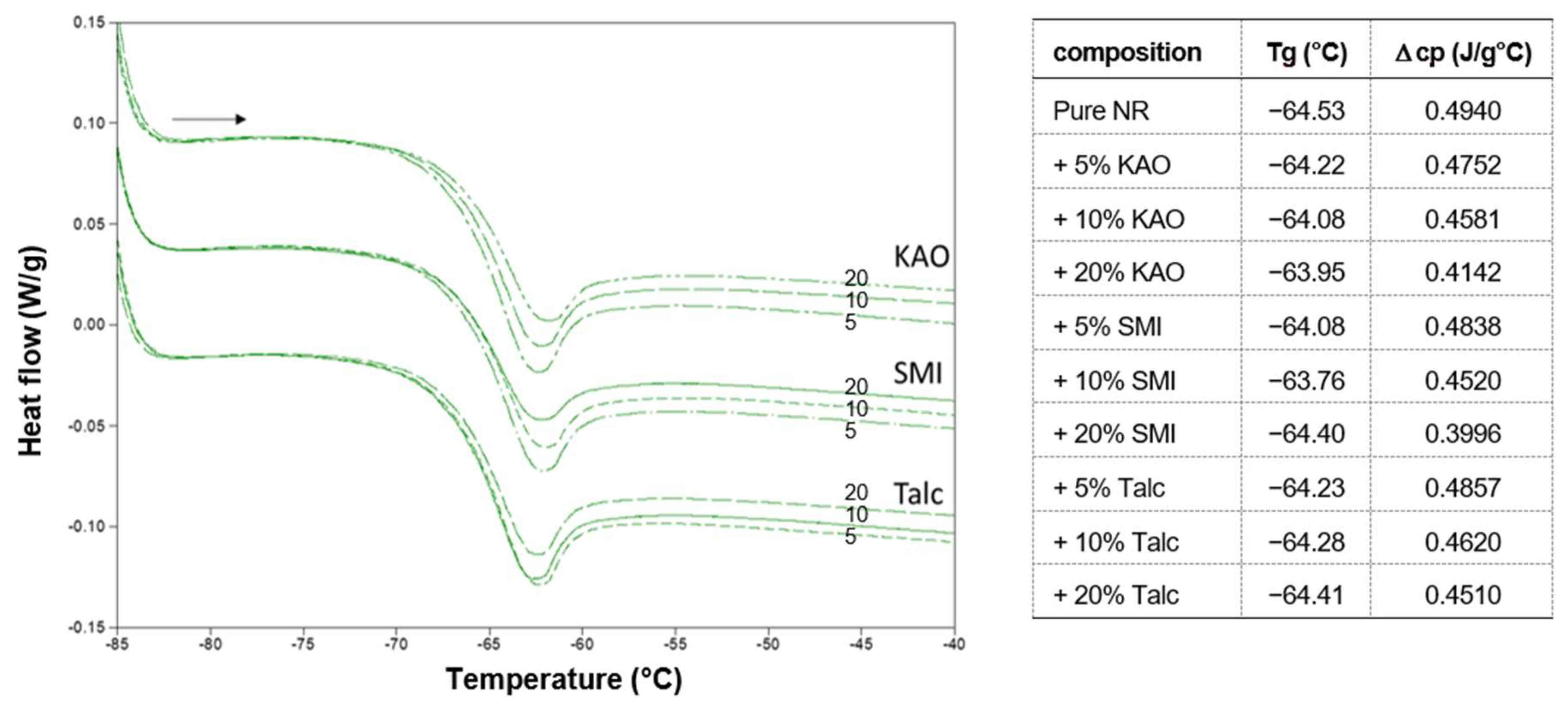
The effects of fillers on the microstructure of the natural rubber latex are evidenced by results of DSC analysis, as summarized in
Figure 2. The pure natural rubber is characterized by a low glass transition temperature of
Tg = −64.53 °C and no further thermal transitions were noticed over the temperature range up to 180 °C as no specific vulcanization agents were added. The change in heat capacity ∆
cp = 0.4940 J/(g°C) over the glass transition is a measure for the change in the molecular mobility in the amorphous phase during the glass transition and may indicate variations in molecular structure induced—e.g., by chain interactions or cross-linking reactions in the amorphous phase.
The slight but consistent variations in
Tg and ∆
cp were noticed in the presence of fillers to different extents, depending on the filler type and concentration. A detail of the glass transition step during heating indeed shows either a shift in the temperature
Tg and/or a reduction in the value ∆
cp in the presence of fillers. The natural rubber composites with fillers show a reduction in ∆
cp, relative to the pure natural rubber, which progressively decreases further as a function of higher filler concentrations: the reduction is the highest for the SMI nanoparticles (to a final value of ∆
cp = 0.3996 J/(g°C)), the lowest for the talc fillers (to a final value of ∆
cp = 0.4510 J/(g°C), and intermediate for the kaolinite fillers (to a final value of ∆
cp = 0.4142 J/(g°C). This would indicate that the fillers assist in creating cross-links between the molecular chains of the natural rubber, preventing molecular mobility during the glass transition. The nanoparticles are indeed most efficient in creating cross-links, likely due to the surface chemistry of the nanoparticles with residual free amic acid groups and imidized moieties, as detailed before [
5], in combination with the nanoscale surface area effect.
The effects of fillers on the structure of natural rubbers are further illustrated from the ATR-FTIR spectra shown in
Figure 3. The spectra both confirm the presence of the fillers in different concentrations in parallel with some changes in the natural latex molecular structure. The FTIR spectra of natural rubbers are characterized by the presence of characteristic bands for cis-1,4-polyisoprene, including 2960 cm
−1 (CH
3 symmetric stretching), 2913, 2852 cm
−1 (CH
2 asymmetric and symmetric stretching), 1655 (-C=C-), 1445 cm
−1 (CH
3 and CH
2 bending), 1376 cm
−1 (CH
3 bending), and 842 cm
−1 (=CH wagging). Apart from that, the spectra of different fillers are characterized by separate absorption bands characteristic for SMI nanoparticles: 1713 cm
−1 (C=O, imide), 701 cm
−1 (aromatic, styrene); kaolinite: 500 to 700 cm
−1 (Si-O), 900 cm
−1 (OH deformation), 1000 cm
−1 (Si-O stretching) 3600 cm
−1 (OH stretching, Al-OH stretching); talc: 672 cm
−1 (Si-O-Si symmetric stretching), 1017 cm
−1 (Si-O-Si asymmetric stretching), and sharp band at 3670 cm
−1 (OH).
The related spectral bands of the fillers are independent of the natural rubber matrix and progressively increase at the higher filler concentrations. A single interaction between the SMI nanoparticles and the matrix can be seen at the shoulder peak around 1360 cm−1: the band is present in pure natural rubbers and gradually intensifies with the higher SMI concentrations, while the band did not appear in single SMI nanoparticles. The latter might indicate physical interactions between the SMI nanoparticles and the CH3 side groups of the natural rubber polymer chain. In addition, an intensified broad peak over the 3200–3500 cm–1 region is most pronounced for the SMI nanoparticle fillers and less present for the kaolinite and talc fillers. This absorption band might be related to the presence of hydroxyl groups that appear to be generated through interactions between the natural rubber with the SMI nanoparticles. On the other hand, no direct changes in the -C=C- double bonds were observed due to chemical cross-linking reactions for either of the fillers. In conclusion, it can be confirmed that strongest physical interactions between the natural rubber matrix and SMI nanoparticles are observed.
3.3. Paper Coating Properties
The morphology of paper surfaces with natural rubber composite coatings are illustrated in
Figure 4, representing top views of the different coating compositions. The pure natural rubber coating was fully flat and covered the paper surface as a smooth polymer film. The aspect of kaolinite fillers is observed as a homogeneous and smooth distribution over the coating surface with progressively more dense coverage at the higher concentrations, while they bring good coating density and likely some topographical roughness effects. The SMI nanoparticles are homogeneously distributed within the coating, causing the creation of small micrometer-scale domains. The talc particles are much rougher and are densely present at the surface in an inhomogeneous distribution over the surface. Due to the platelet morphology of talc particles, they are randomly oriented at the surface either perpendicularly sticking out or embedded parallel to the surface.
The results of surface properties, including wetting and adhesive properties, are summarized in bar charts of
Figure 5. The static contact angle values of water and diiodomethane in
Figure 5a show slight and consistent variations among the different coating types and filler concentrations, relative to the pure natural rubber coating. The contact angles remained stable on the coatings for about 15 seconds, except for the pure natural rubber, as the homogeneity and coverage of the coating was not perfect and fully continuous for the pure natural rubber coating. The exposure of paper fibers at the surface created voids for the flow of the water through the coating, while the presence of fillers improved the coating coverage and density, providing better bulkiness compared to the pure natural rubber. The original rubber coating had a water contact angle of 95°, being in the hydrophobic range. The presence of kaolinite gradually increases the coating hydrophobicity, likely due to the hydrophobic properties of the fillers in combination with the creation of some additional surface roughness, seen in the microscopic images. The talc particles are hydrophilic, and their properties consequently prevail while exposed at the surface, resulting in a gradual decrease in hydrophobicity with the higher talc concentrations. The hydrophobic properties of SMI nanoparticles are beneficially exploited while added in different concentrations, rising up to a maximum water contact angle of 110°. While the water contact angle indicates the polar interactions, the diiodomethane is an apolar liquid and often shows opposite trends to the water contact angles.
The adhesion between coated surfaces of natural rubber composites has been studied in the frame of the tendency for self-adhesion of natural rubber materials. The work of adhesion, Wa = γ
L (1 + cos
θ) with γ
L = liquid tension, and taking into account the contact between similar rubber coating materials, can theoretically be calculated from the water and diiodomethane contact angles. The calculated values are represented in
Figure 5b, where it can be noticed that the predicted adhesion varies for the different coating types. The theoretical adhesion is highest for the pure natural rubber and is lower in the presence of filler materials: with increasing filler concentrations, the adhesion gradually decreases in the presence of kaolinite fillers and SMI nanoparticles, while the adhesion increases in the presence of talc particles. This can indeed be related to the hydrophobic effect of the kaolinite and SMI nanoparticles and the hydrophilic effect of the talc particles. The results of experimental adhesion measurements from a loop test with contact between similar rubber composite materials is represented in
Figure 5c, and confirm the trends from theoretical calculations. From that, it is mainly revealed that the tendency for adhesion of rubber composite coatings can be predicted from water contact angle measurements and is steered by the hydrophobicity of the coated surface.