Calcium-Associated Anions Play a Dual Role in Modulating Cadmium Uptake and Translocation in Wheat
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Growing Conditions
2.2. Elemental Analyses
2.3. Statistical Analysis
3. Results
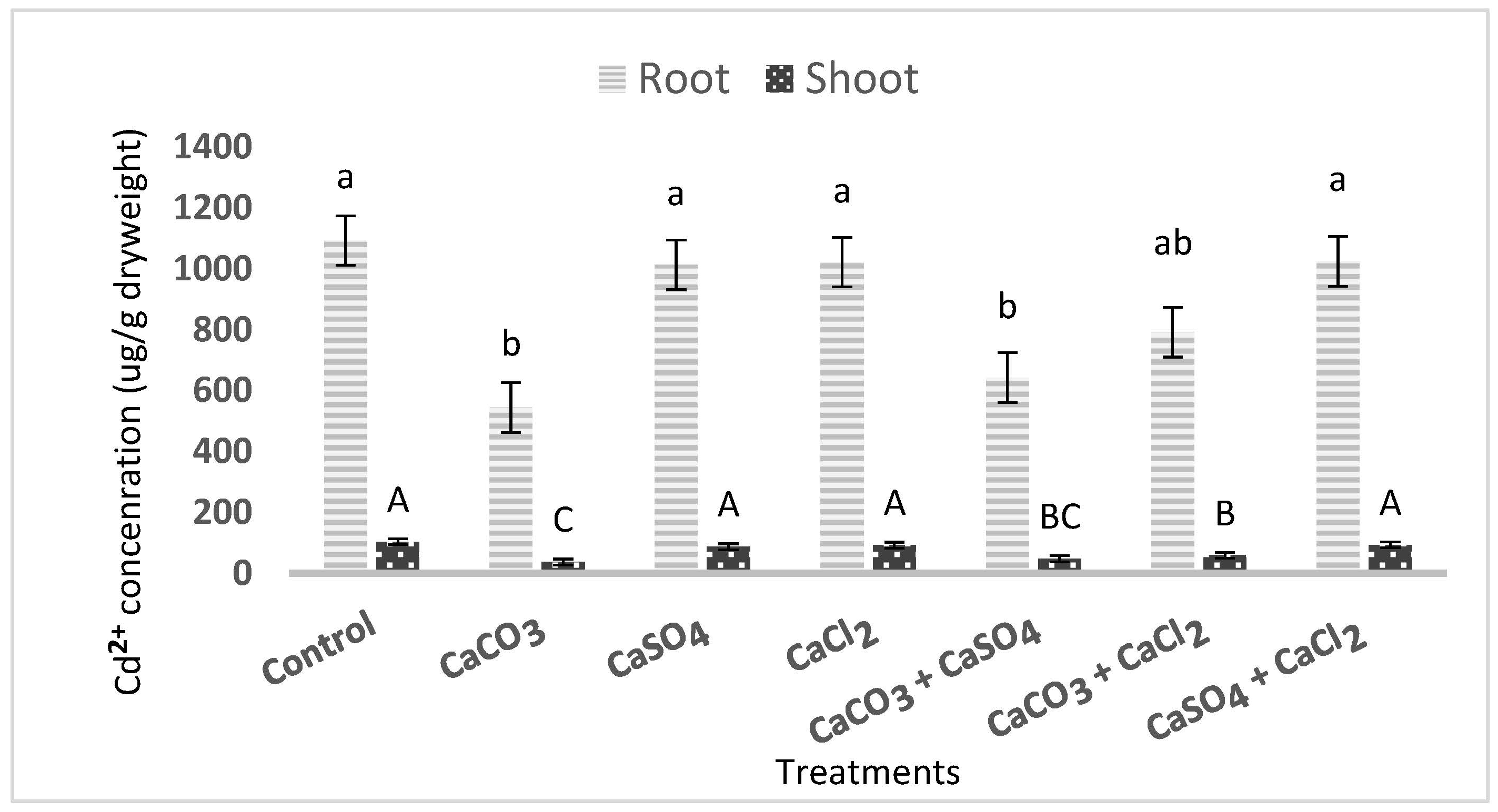
3.1. Effect of Ca2+ Treatments on Cd2+ Uptake and Translocation in Wheat
3.1.1. Effect of Ca2+ Treatments on pH of Nutrition Solution
3.1.2. Effect of Calcium Treatments on Root and Shoot Fresh Biomass
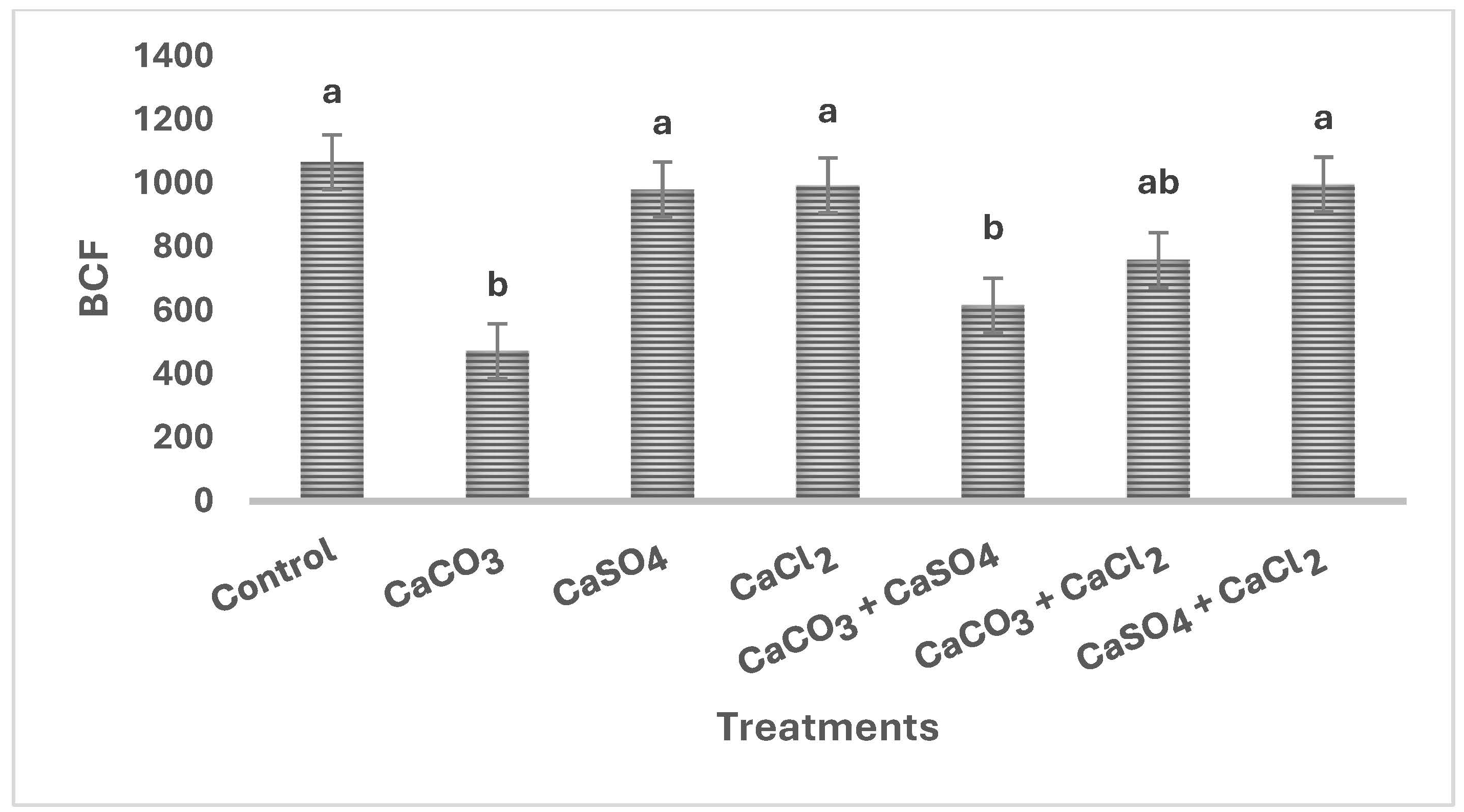
3.1.3. Effect of Ca2+ Treatments on Cd2+ Concentration in Wheat and Bioconcentration Factor
3.2. Effect of Ca2+ Treatments on Nutrient Uptake
4. Discussion
4.1. Effect of Ca2+ Treatments on Cd2+ Uptake and Translocation in Wheat
4.2. Effect of Ca2+ Treatments on Nutrient Uptake
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, J.; Wang, C.; Qi, L. Phosphorus is more effective than nitrogen in restoring plant communities of heavy metals polluted soils. Environ. Pollut. 2020, 266, 115259. [Google Scholar] [CrossRef] [PubMed]
- Fatima, G.; Raza, A.; Hadi, R.; Hadi, N.; Mahdi, A.A. Cadmium in human diseases: It’s more than just a mere metal. Indian J. Clin. Biochem. 2019, 34, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Järup, L.; Akesson, A. Current status of cadmium as an environmental health problem. Toxicol. Appl. Pharmacol. 2009, 238, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Clemens, S.; Aarts, M.; Thomine, S.; Verbruggen, N. Plant science: The key to preventing slow cadmium poisoning. Trends Plant Sci. 2013, 18, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Rahim, H.U.; Akbar, W.A.; Alatalo, J.M. A Comprehensive Literature Review on Cadmium (Cd) Status in the Soil Environment and Its Immobilization by Biochar-Based Materials. Agronomy 2022, 12, 877. [Google Scholar] [CrossRef]
- Bolan, N.S.; Adriano, D.C.; Mani, P.A.; Duraisamy, A. Immobilization and phyto availability of cadmium in variable charge soils. II. Effect of lime addition. Plant Soil 2003, 251, 187–198. [Google Scholar] [CrossRef]
- Rehman, M.Z.; Rizwan, M.; Ghafoor, A.; Naeem, A.; Ali, S.; Sbir, M.; Qayyum, M.F. Effect of inorganic amendments for in situ stabilization of cadmium in contaminated soils and its phyto-availability to wheat and rice under rotation. Environ. Sci. Pollut. Res. 2015, 22, 16897–16906. [Google Scholar] [CrossRef] [PubMed]
- Hailu, B.; Estifanos, S. Investigating the effect of gypsum powder on chemical constituents of soil and selected crops, Adigudem, Tigray, Ethiopia. Momona Ethiop. J. Sci. 2021, 13, 164–176. [Google Scholar] [CrossRef]
- Wu, J.; Sagervanshi, A.; Mühling, K.H. Sulfate facilitates cadmium accumulation in leaves of Vicia faba L. at flowering stage. Ecotoxicol. Environ. Saf. 2018, 156, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Argüello, D.; Dekeyrel, J.; Chavez, E.; Smolders, E. Gypsum application lowers cadmium uptake in cacao in soils with high cation exchange capacity only: A soil chemical analysis. Soil Sci. 2022, 73, e13230. [Google Scholar] [CrossRef]
- He, Z.Z.; Chai, M.; Wei, Y. Effects of CaCl2 on the Cd accumulation and stress of Spartina alterniflora. Chin. J. Ecol. 2013, 32, 1571–1577. [Google Scholar]
- Mei, X.; Li, S.; Li, Q.; Yang, Y.; Luo, X.; He, B.; Li, H.; Xu, Z. Sodium chloride salinity reduces Cd uptake by edible amaranth (Amaranthus mangostanus L.) via competition for ca channels. Ecotoxicol. Environ. Saf. 2014, 105, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, N.; Tomioka, R.; Takenaka, C. Effects of calcium on cadmium uptake and transport in the tree species Gamblea innovans. Soil Sci. Plant Nutr. 2011, 57, 691–695. [Google Scholar] [CrossRef][Green Version]
- Nazar, R.; Iqbal, N.; Masood, A.; Khan, M.I.R.; Syeed, S.; Khan, N.A. Cadmium toxicity in plants and role of mineral nutrients in its alleviation. Am. J. Plant Sci. 2012, 3, 1476–1489. [Google Scholar] [CrossRef]
- Roth, U.; Roepenack-Lahaye, E.V.; Clemens, S. Proteome changes in arabidopsis thaliana roots upon exposure to Cd2+. J. Exp. Bot. 2006, 57, 4003–4013. [Google Scholar] [CrossRef] [PubMed]
- Papoyan, A.; Kochian, L.V. Identification of Thlaspicaerulescens Genes That May Be Involved in heavy metal hyperaccumulation and tolerance. Characterization of a novel heavy metal transporting ATPase. Plant Physiol. 2004, 136, 3814–3823. [Google Scholar] [CrossRef] [PubMed]
- Ali, U.; Zhong, M.; Shar, T.; Fiaz, S.; Xie, L.; Jiao, G.; Ahmad, S.; Sheng, Z.; Tang, S.; Wei, X.; et al. The influence of pH on cadmium accumulation in seedlings of rice (Oryza sativa L.). J. Plant Growth Regul. 2020, 39, 930–940. [Google Scholar] [CrossRef]
- Liu, J.; Feng, X.; Qiu, G.; Li, H.; Wang, Y.; Chen, X.; Fu, Q.; Guo, B. Inhibition roles of calcium in cadmium uptake and translocation in rice: A Review. Int. J. Mol. Sci. 2023, 24, 11587. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Liu, H.; Nie, Z.; Rengel, Z.; Gao, W.; Li, C.; Zhao, P. Toxicity of cadmium and its competition with mineral nutrients for uptake by plants: A review Shiyu. Pedosphere 2020, 30, 168–180. [Google Scholar] [CrossRef]
- Manzoor, M.; Abdalla, M.A.; Hussain, M.A.; Mühling, K.H. Silicon-Selenium interplay imparts cadmium resistance in wheat through an up-regulating antioxidant system. Int. J. Mol. Sci. 2024, 25, 387. [Google Scholar] [CrossRef] [PubMed]
- Mourato, M.; Pinto, F.; Moreira, I.; Sales, J.; Leitão, I. The Effect of Cd stress in mineral nutrient uptake in plants. In Cadmium Toxicity and Tolerance in Plants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 327–348. [Google Scholar] [CrossRef]

| Treatment | pH |
|---|---|
| Control | 6.62 ± 0.02 c |
| CaCO3 | 7.82 ± 0.03 a |
| CaSO4 | 6.44 ± 0.02 d |
| CaCl2 | 5.78 ± 0.04 f |
| CaCO3 + CaSO4 | 6.72 ± 0.02 b |
| CaCO3 + CaCl2 | 6.79 ± 0.02 b |
| CaSO4 + CaCl2 | 6.30 ± 0.04 d |
| Cd2+ Concentration | BCF | TF | ||
|---|---|---|---|---|
| Root | Shoot | |||
| pH | −0.57 ** | −0.65 ** | −0.59 ** | −0.25 ns |
| Ca2+ | −0.26 ns | −0.40 * | −0.28 ns | −0.41 * |
| CO32+ | −0.69 ** | −0.87 ** | −0.71 ** | −0.47 * |
| SO42+ | 0.07 ns | 0.05 ns | 0.07 ns | −0.08 ns |
| Cl− | 0.23 ns | 0.24 ns | 0.24 ns | −0.004 ns |
| Cd2+ | CdCl+ | CdEDTA | CdSO4 (p) | CdCO3 (p) | Cd(CO3)2 | |
|---|---|---|---|---|---|---|
| Control | 62.14 | 14.98 | 16.18 | 5.94 | 0 | 0 |
| CaCO3 | 4.89 | 1.11 | 18.32 | 0.40 | 41.59 | 33.60 |
| CaSO4 | 56.19 | 11.98 | 16.19 | 14.35 | 0 | 0 |
| CaCl2 | 49.10 | 30.21 | 15.96 | 3.25 | 0 | 0 |
| CaCO3 + CaSO4 | 7.13 | 1.48 | 18.47 | 1.68 | 42.18 | 28.89 |
| CaCO3 + CaCl2 | 7.86 | 4.70 | 18.41 | 0.48 | 42.07 | 26.22 |
| CaSO4 + CaCl2 | 46.68 | 26.44 | 15.98 | 9.23 | 0 | 0 |
| Uptake | |||
|---|---|---|---|
| Mg2+ | Mn2+ | Cu2+ | |
| Control | 2.45 ± 0.7 b | 101 ± 28 c | 15 ± 5 c |
| CaCO3 | 5.39 ± 0.6 ab | 942 ± 32 a | 70 ± 27 a |
| CaSO4 | 1.79 ± 0.4 b | 53 ± 8 c | 37 ± 10 bc |
| CaCl2 | 1.69 ± 0.3 b | 51 ± 4 c | 28 ± 11 bc |
| CaCO3 + CaSO4 | 9.01 ± 0.6 a | 820 ± 32 a | 82 ± 29 a |
| CaCO3 + CaCl2 | 3.13 ± 0.9 b | 471 ± 20 b | 54 ± 2 ab |
| CaSO4 + CaCl2 | 1.69 ± 0.3 b | 65 ± 9 c | 31 ± 3 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safari Sinegani, M.; Manzoor, M.; Mühling, K.H. Calcium-Associated Anions Play a Dual Role in Modulating Cadmium Uptake and Translocation in Wheat. Pollutants 2024, 4, 340-349. https://doi.org/10.3390/pollutants4030023
Safari Sinegani M, Manzoor M, Mühling KH. Calcium-Associated Anions Play a Dual Role in Modulating Cadmium Uptake and Translocation in Wheat. Pollutants. 2024; 4(3):340-349. https://doi.org/10.3390/pollutants4030023
Chicago/Turabian StyleSafari Sinegani, Mahboobe, Maria Manzoor, and Karl Hermann Mühling. 2024. "Calcium-Associated Anions Play a Dual Role in Modulating Cadmium Uptake and Translocation in Wheat" Pollutants 4, no. 3: 340-349. https://doi.org/10.3390/pollutants4030023
APA StyleSafari Sinegani, M., Manzoor, M., & Mühling, K. H. (2024). Calcium-Associated Anions Play a Dual Role in Modulating Cadmium Uptake and Translocation in Wheat. Pollutants, 4(3), 340-349. https://doi.org/10.3390/pollutants4030023







