Quantifying Zinc Contamination from Laboratory Syringes
Abstract
1. Introduction
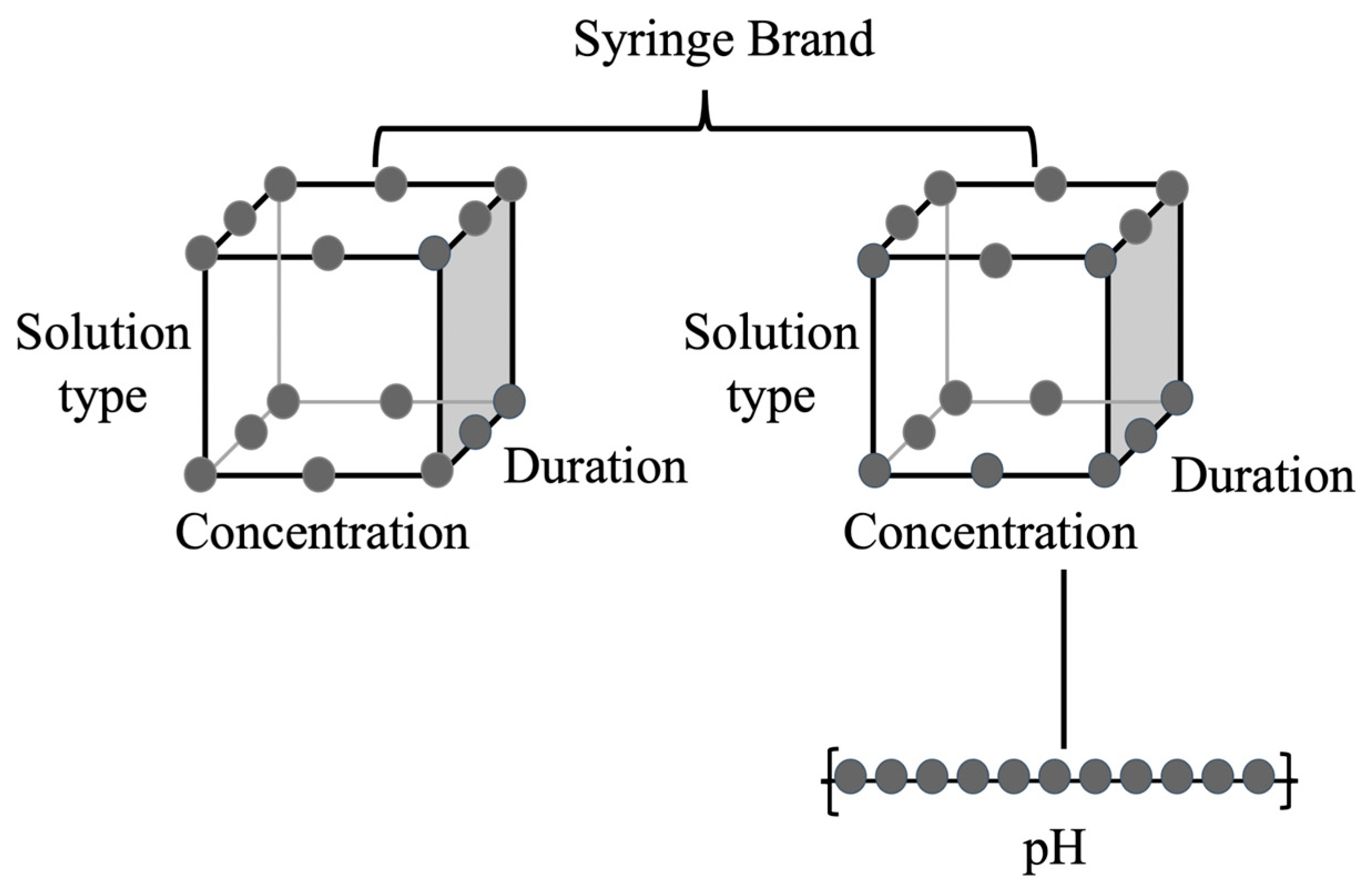
2. Material and Methods
2.1. Solution Preparation
2.2. Solution Exposure (in Syringe)
3. ICP-MS Methods
4. Results
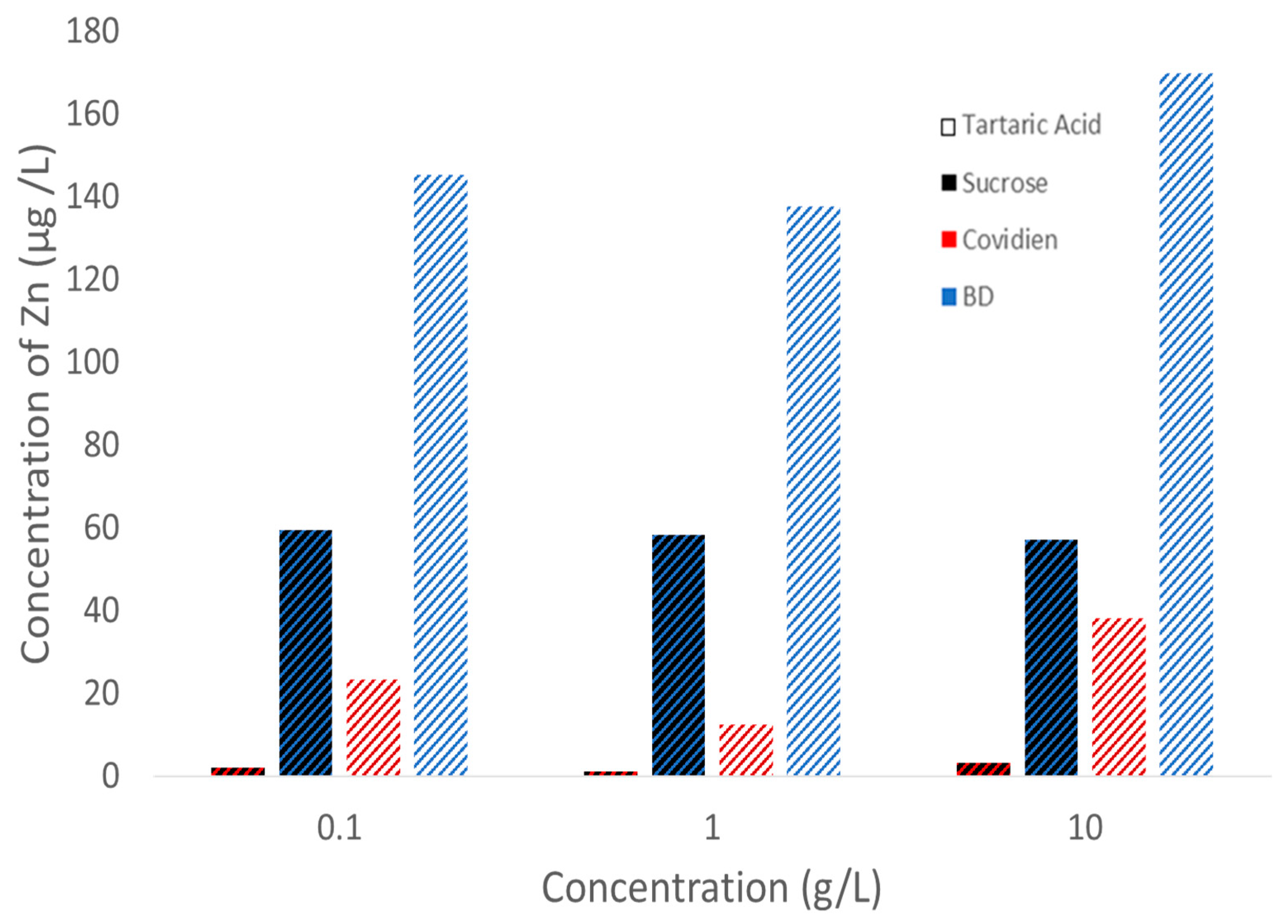
4.1. Factor: Solution Type/Concentration
4.2. Factor: Syringe Brand
4.3. Factor: Time
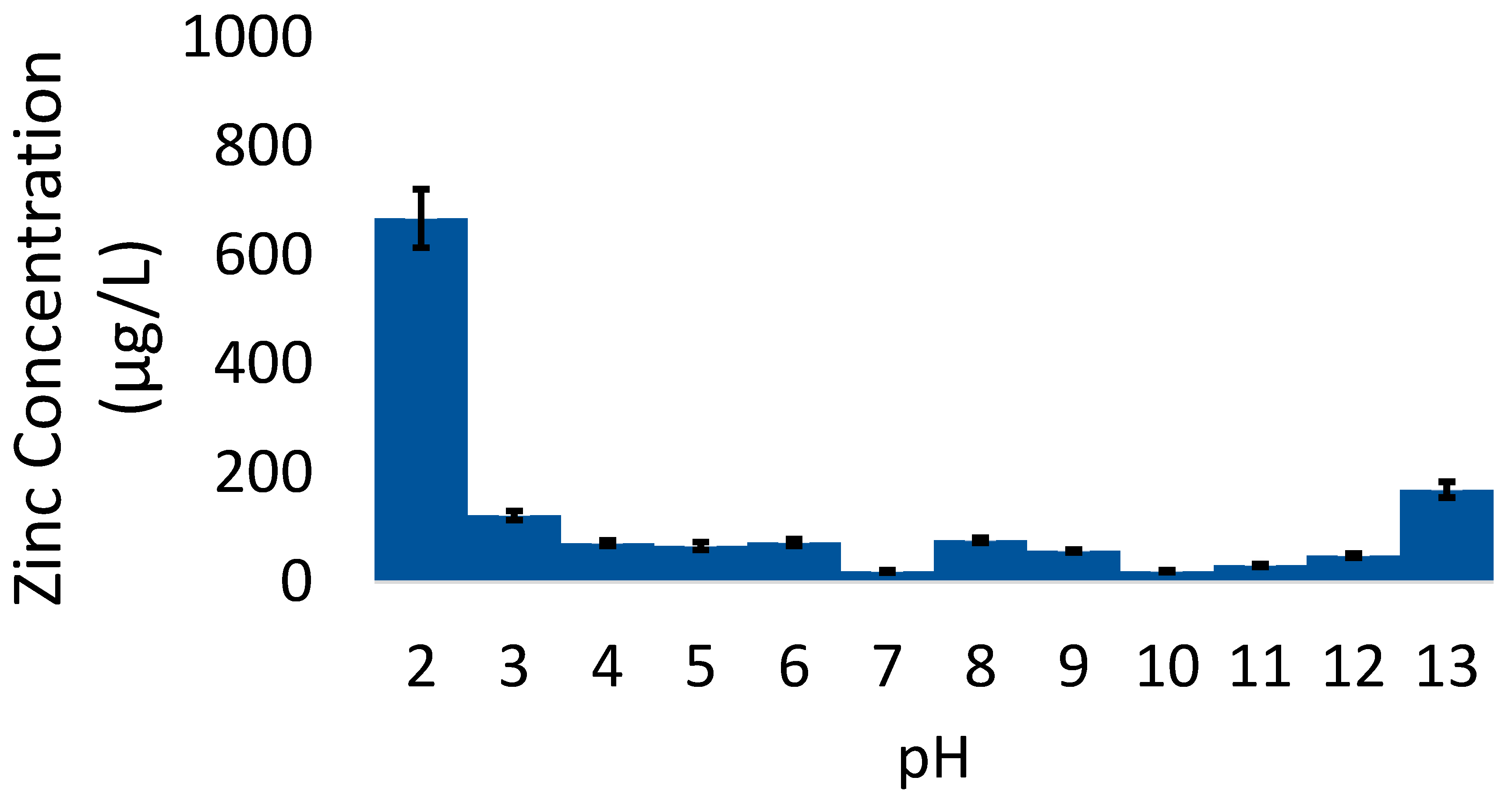
4.4. Factor: pH
5. Discussion and Conclusions
- pH;
- Syringe brand;
- Time;
- Solution type and concentration.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boerner, L.K. Metal-free? The mistake that chemists seem doomed to repeat. Chem. Eng. News 2022, 100, 20. [Google Scholar] [CrossRef]
- Millipore Sigma. Zinc in Cell Culture. Available online: https://www.sigmaaldrich.com/US/en/technical-documents/technical-article/cell-culture-and-cell-culture-analysis/mammalian-cell-culture/zinc (accessed on 11 April 2024).
- PATH. The Syringe Shortage Explained. Available online: https://www.path.org/our-impact/articles/syringe-shortage-explained/ (accessed on 24 March 2022).
- UN News. COVID-19 Pandemic Brings Global Syringe Shortage into Sharp Focus. Available online: https://news.un.org/en/story/2021/11/1105362 (accessed on 24 March 2022).
- Heideman, G.; Datta, R.N.; Noordermeer, J.W.M.; Baarle, B.V. Influence of zinc oxide During Different Stages of Sulfur Vulcanization. Elucidated by Model Compound Studies. J. Appl. Polym. Sci. 2005, 95, 1388–1404. [Google Scholar] [CrossRef]
- Taylor, A.; Marks, V. Contamination from Syringes and Blood Container Pots in Trace Element Analysis. Ann. Clin. Biochem. 1973, 10, 42–46. [Google Scholar] [CrossRef]
- Song, M. An Introduction to Prefilled Syringe Selection—Needle-Free and Dual-Chamber Devices. Pharmaceutical Online, 30 September 2019. Available online: https://www.pharmaceuticalonline.com/doc/an-introduction-to-prefilled-syringe-selection-needle-free-and-dual-chamber-devices-0001 (accessed on 24 March 2022).
- Houk, R.S.; Fassel, V.A.; Flecsh, G.D.; Svec, H.J.; Gray, A.L.; Taylor, C.E. Inductively coupled argon plasma as an ion source for mass spectrometric determination of trace elements. Anal. Chem. 1980, 52, 2283–2289. [Google Scholar] [CrossRef]
- Perkin Elmer. The 30-Minute Guide to ICP-MS. Available online: https://resources.perkinelmer.com/corporate/pdfs/downloads/tch_icpmsthirtyminuteguide.pdf (accessed on 24 March 2022).
- Radboud University. ICP-MS. Available online: https://www.ru.nl/science/gi/facilities-activities/elemental-analysis/icp-ms/ (accessed on 24 March 2022).
- Thermo Fisher Scientific. Thermo Scientific iCAP RQ ICP-MS. 2017. Available online: https://assets.thermofisher.com/TFS-Assets/CMD/brochures/BR-43317-ICP-MS-iCAP-RQ-BR43317-EN.pdf (accessed on 24 March 2022).
- Bowring, S.; Boyle, E.; Chatterjee, N.; Dudas, F. Interferences in Inductively Coupled Plasma Mass Spectrometry; MIT: Cambridge MA, USA, 2011. [Google Scholar]
- Neubauer, K. Reducing the Effects of Interferences in Quadrupole ICP-MS. Spectroscopy, 1 November 2020. Available online: https://www.spectroscopyonline.com/view/reducing-effects-interferences-quadrupole-icp-ms (accessed on 24 March 2022).
- Evans, H.; Giglio, J. Interferences in inductively coupled plasma mass spectrometry—A Review. J. Anal. At. Spectrom. 1993, 8, 1–18. [Google Scholar] [CrossRef]
- Gossens, J.; Dams, R. Anion exchange for the elimination of spectral interference caused by chlorine and sulfur in inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 1992, 7, 1167–1171. [Google Scholar] [CrossRef]
- Laborda, F.; Baxter, M.J.; Crews, H.M.; Dennis, J. Reduction of polyatomic interferences in inductively coupled plasma mass spectrometry by selection of instrumental parameters and using an argon-nitrogen plasma: Effect on multi-element analyses. J. Anal. At. Spectrom. 1994, 9, 727–736. [Google Scholar] [CrossRef]
- Longbottom, J.E.; Martin, T.D.; Edgell, K.W.; Long, S.E.; Plantz, M.R.; Warden, B.E. Determination of trace elements in water by inductively coupled-plasma spectrometry: Collaborative study. J. AOAC 1994, 77, 1004–1023. [Google Scholar] [CrossRef]
- Malin, J.N.; Hayes, P.L.; Geiger, F.M. Interactions of Ca, Zn, and Cd Ions at Buried Solid/Water Interfaces Studied by Second Harmonic Generation. J. Phys. Chem. 2009, 113, 2041–2052. [Google Scholar] [CrossRef]
- McLaren, J.W.; Beauchemin, D.; Berman, S.S. Application of isotope dilution inductively coupled plasma mass spectrometry to the analysis of marine sediments. Anal. Chem. 1987, 59, 610–613. [Google Scholar] [CrossRef]
- Platzner, I.; Sala, J.V.; Mousty, F.; Trincherini, P.R.; Polettini, A.L. Signal enhancement and reduction of interferences in inductively coupled plasma mass spectrometry with an argon-trifluoromethane mixed aerosol carrier gas. J. Anal. At. Spectrom. 1994, 9, 719–726. [Google Scholar] [CrossRef]
- Tan, S.H.; Horlick, G. Background spectral features in inductively coupled plasma/mass spectrometry. Appl. Spectrosc. 1986, 40, 445–460. [Google Scholar] [CrossRef]
- Vandecasteele, C.; Vanhoe, H.; Dams, R. Inductively coupled plasma mass spectrometry of biological samples. J. Anal. At. Spectrom. 1993, 8, 781–786. [Google Scholar] [CrossRef]
- Oehlert, G.W. A First Course in Design and Analysis of Experiments; Creative Commons: Mountain View, CA, USA, 2010. [Google Scholar]
- Steer, J.M.; Griffiths, A.J. Investigation of Carboxylic Acids and Non-Aqueous Solvents for the Selective Leaching of Zinc from Blast Furnace Dust Slurry. Hydrometallurgy 2013, 140, 34–41. [Google Scholar] [CrossRef]
- Moreno, J.; Peinado, R. Chapter 8–Carboxylic Acids: Structure and Properties. In Enological Chemistry; Academic Press: Cambridge, MA, USA, 2012; pp. 109–120. [Google Scholar]
- Bala, T.; Prasad, B.L.V.; Sastry, M.; Kahaly, M.; Waghmare, U.V. Interaction of Different Metal Ions with Carboxylic Acid Group: A Quantitative Study. J. Phys. Chem. 2007, 111, 6183–6190. [Google Scholar] [CrossRef] [PubMed]

| Item | Supplier | Lot Number (s) |
|---|---|---|
| Covidien 60 mL Luer-Lok syringe | VWR (Radnor, PA, USA) | 027252X |
| BD 50 mL Luer-Lok syringe | VWR (Radnor, PA, USA) | 1145497 and 1215381 |
| Norm-Jet 60 mL Luer-Lok syringe * | VWR (Radnor, PA, USA) | 20|18 0 B |
| Sucrose | VWR (Radnor, PA, USA) | 20G1356994 |
| L-tartaric acid | VWR (Radnor, PA, USA) | 19J1056043 |
| 25 mm 0.45 μm nylon membrane | VWR (Radnor, PA, USA) | 10163004A |
| Syringe filter | ||
| 15 mL PP centrifuge tubes | VWR (Radnor, PA, USA) | 21024058 |
| Multi-element standard solution | Inorganic Ventures (Christiansburg, VA, USA) | P2-MEB682937 |
| Ultrapure water (18 MΩ) | Veolia (Aubervilliers, France) | N/A |
| Sodium hydroxide | VWR (Radnor, PA, USA) | Bulk |
| Nitric Acid—low metals | Aristar Plus/VWR (Radnor, PA, USA) | 1121050 |
| ICP-MS Plasma | Parameters |
|---|---|
| Instrument Parameters | |
| RF power | 1550 W |
| Plasma power | 1550 W |
| Auxiliary gas flow | 0.80 L min−1 |
| Nebulizer gas flow | 1.04 L min−1 |
| Sampling depth | 5 mm |
| Sample/skimmer diameter orifice | Nickel cones/3.5 mm insert |
| Acquisition parameters | |
| Scanning mode | KED |
| Dwell time | 0.01 s |
| Sampling flow | 20 rpm |
| Replicate count | 15 |
| Integration mode | Peak area |
| Isotopes | 64Zn, 66Zn, and 68Zn |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lindgren, S.G.; Sakol, L.J.; Hoover, M.; Raymond, T.M.; Dutcher, D.D. Quantifying Zinc Contamination from Laboratory Syringes. Pollutants 2024, 4, 350-358. https://doi.org/10.3390/pollutants4030024
Lindgren SG, Sakol LJ, Hoover M, Raymond TM, Dutcher DD. Quantifying Zinc Contamination from Laboratory Syringes. Pollutants. 2024; 4(3):350-358. https://doi.org/10.3390/pollutants4030024
Chicago/Turabian StyleLindgren, Sarah G., Laura J. Sakol, Monica Hoover, Timothy M. Raymond, and Dabrina D. Dutcher. 2024. "Quantifying Zinc Contamination from Laboratory Syringes" Pollutants 4, no. 3: 350-358. https://doi.org/10.3390/pollutants4030024
APA StyleLindgren, S. G., Sakol, L. J., Hoover, M., Raymond, T. M., & Dutcher, D. D. (2024). Quantifying Zinc Contamination from Laboratory Syringes. Pollutants, 4(3), 350-358. https://doi.org/10.3390/pollutants4030024







