Study of Corrosion Protection of Concrete in Sewage Systems with Magnesium Hydroxide Coatings †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Concrete Preparation
2.2. Preparation and Application of Coatings
2.3. Pull-Off
2.4. Acid Spraying
2.5. XRD Analysis
3. Results
3.1. Pull-Off
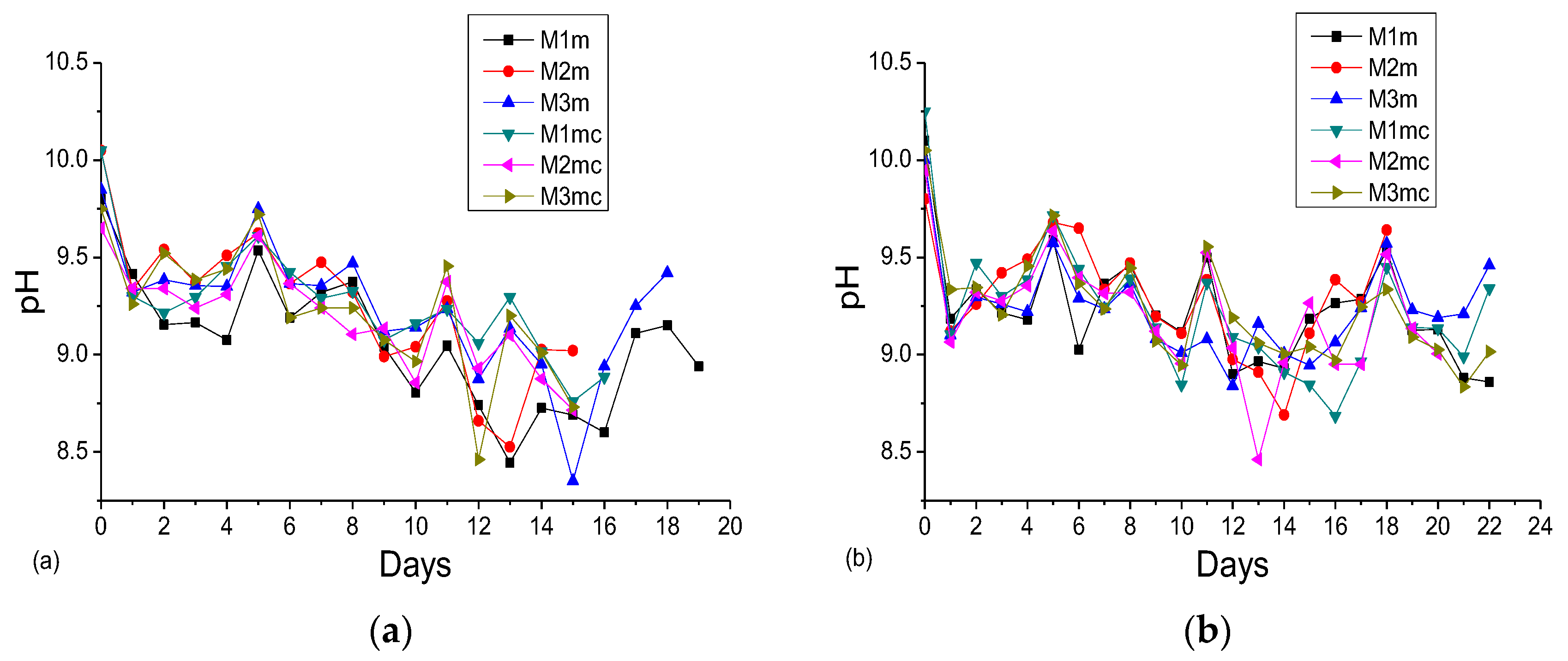
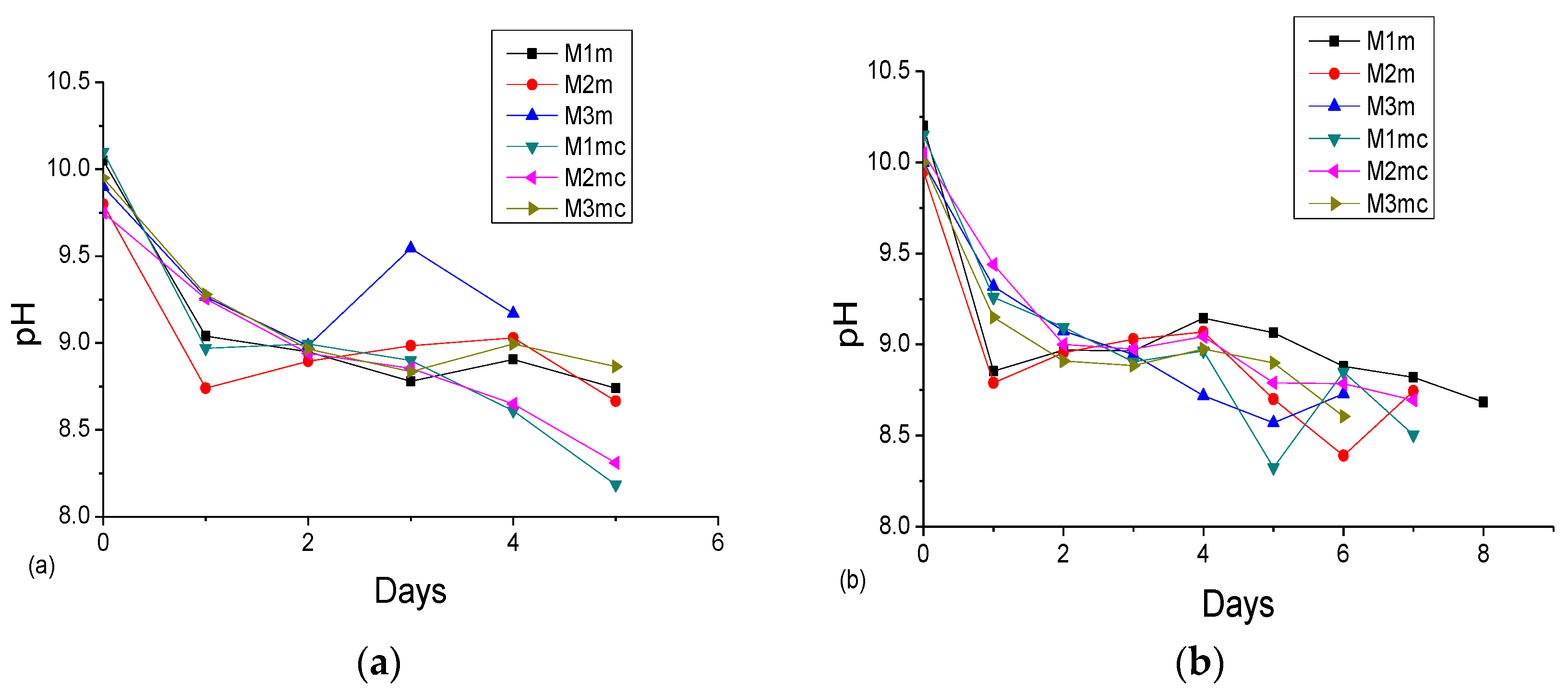
3.2. Accelerated Acid Spraying Test
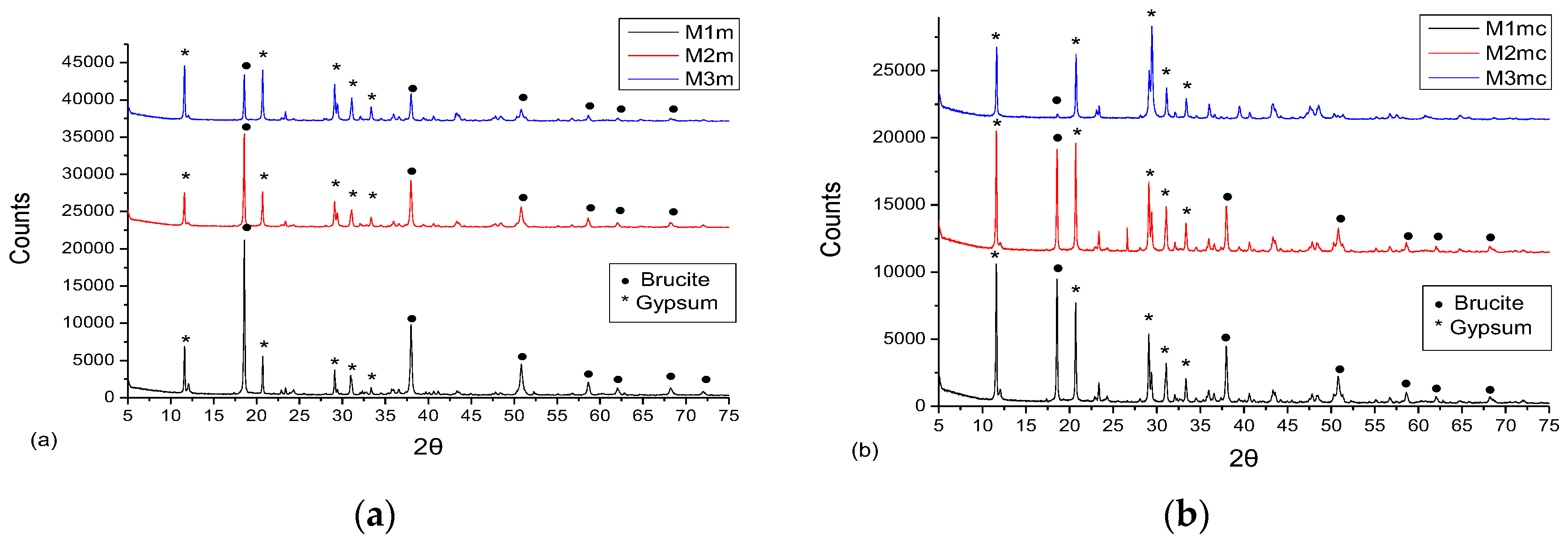
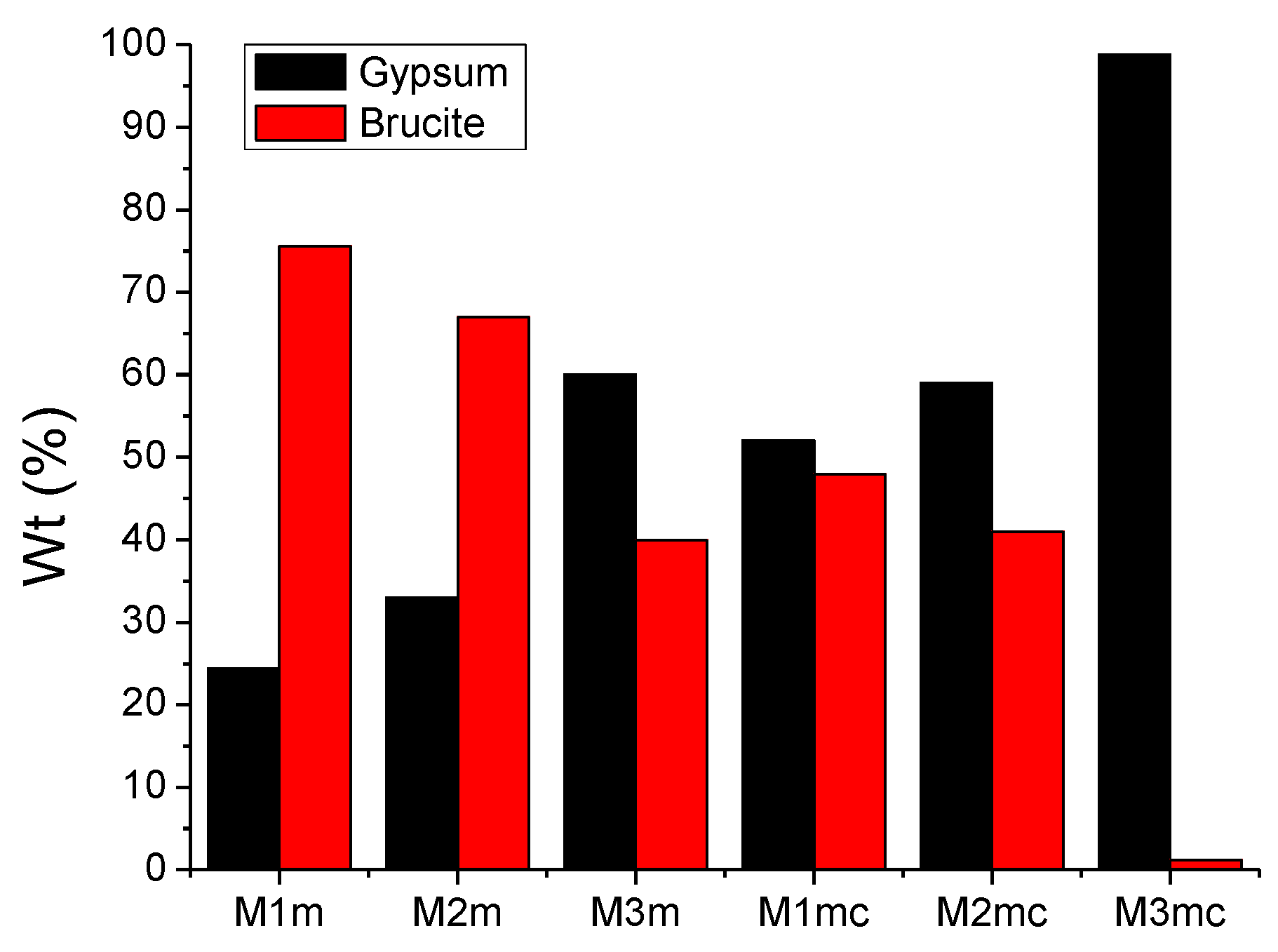
3.3. XRD Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments

Conflicts of Interest
References
- Hvitved-Jacobsen, T.; Vollertsen, J.; Nielsen, A.H.; Vollertsen, J.; Nielsen, A.H. Sewer Processes : Microbial and Chemical Process Engineering of Sewer Networks; CRC Press: Boca Raton, FL, USA, 2013; p. 371. [Google Scholar]
- ASCE (American Society of Civil Engineers); WPCF (Water Pollution Control Federation); ASCE (American Society of Civil Engineers); WPCF (Water Pollution Control Federation). Gravity Sanitary Sewer Design and Construction; Bizier, P., Ed.; American Society of Civil Engineers: Reston, VA, USA, 2007. [Google Scholar]
- Mori, T.; Koga, M.; Hikosaka, Y.; Nonaka, T.; Mishina, F.; Sakai, Y.; Koizumi, J. Microbial corrosion of concrete sewer pipes, H2S production from sediments and determination of corrosion rate. Water Sci. Technol. 1991, 23, 1275–1282. [Google Scholar] [CrossRef]
- Mori, T.; Nonaka, T.; Tazaki, K.; Koga, M.; Hikosaka, Y.; Noda, S.; Mori, T.; Nonaka, T.; Tazaki, K.; Koga, M.; et al. Interactions of nutrients, moisture and pH on microbial corrosion of concrete sewer pipes. Water Res. 1992, 26, 29–37. [Google Scholar] [CrossRef]
- Herisson, J.; Guéguen-Minerbe, M.; van Hullebusch, E.D.; Chaussadent, T.; Herisson, J.; Guéguen-Minerbe, M.; van Hullebusch, E.D.; Chaussadent, T. Influence of the binder on the behaviour of mortars exposed to H2S in sewer networks: A long-term durability study. Mater. Struct. 2017, 50, 8. [Google Scholar] [CrossRef]
- Vincke, E.; Van Wanseele, E.; Monteny, J.; Beeldens, A.; De Belie, N.; Taerwe, L.; Van Gemert, D.; Verstraete, W.; et al. Influence of polymer addition on biogenic sulfuric acid attack of concrete. Int. Biodeterior. Biodegrad. 2002, 49, 283–292. [Google Scholar] [CrossRef]
- Aguiar, J.B.; Camões, A.; Moreira, P.M.; Aguiar, J.B.; Camões, A.; Moreira, P.M. Coatings for Concrete Protection against Aggressive Environments. J. Adv. Concr. Technol. 2008, 6, 243–250. [Google Scholar] [CrossRef]
- Roghanian, N.; Banthia, N.; Roghanian, N.; Banthia, N. Development of a sustainable coating and repair material to prevent bio-corrosion in concrete sewer and waste-water pipes. Cem. Concr. Compos. 2019, 100, 99–107. [Google Scholar] [CrossRef]
- Gutierrez, O.; Park, D.; Sharma, K.R.; Yuan, Z.; Gutierrez, O.; Park, D.; Sharma, K.R.; Yuan, Z. Effects of long-term pH elevation on the sulfate-reducing and methanogenic activities of anaerobic sewer biofilms. Water Res. 2009, 43, 2549–2557. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Keller, J.; Yuan, Z.; Zhang, L.; Keller, J.; Yuan, Z. Ferrous salt demand for sulfide control in rising main sewers: Tests on a laboratory-scale sewer system. J. Environ. Eng. 2010, 136, 1180–1187. [Google Scholar] [CrossRef]
- Ganigue, R.; Gutierrez, O.; Rootsey, R.; Yuan, Z.; Ganigue, R.; Gutierrez, O.; Rootsey, R.; Yuan, Z. Chemical dosing for sulfide control in Australia: An industry survey. Water Res. 2011, 45, 6564–6574. [Google Scholar] [CrossRef] [PubMed]
- Sydney, R.; Esfandi, E.; Surapaneni, S.; Sydney, R.; Esfandi, E.; Surapaneni, S. Control Concrete Sewer Corrosion via the Crown Spray Process. Water Environ Res. 1996, 68, 338–347. [Google Scholar] [CrossRef]
- European Committee for Standardization. EN 1766:2000 Products and Systems for the Protection and Repair of Concrete Structures. Test Methods. Reference Concretes for Testing; European Standard: Brussels, Belgium, 2000. [Google Scholar]
- European Committee for Standarization. 196-1:1995: Methods of Testing Cement—Part 1: Determination of Strength; European Committee for Standarization: Brussels, Belgium, 1995. [Google Scholar]
- European Committee for Standarization. ΕΝ 1542:1999: Products and Systems for the Protection and Repair of Concrete Structures—Test Methods—Measurement of bond Strength by Pull-Off; European Committee for Standarization: Brussels, Belgium, 1999. [Google Scholar]
- European Committee for Standarization. BS ΕΝ 13578:2003: Products and Systems for the Protection and Repair of Concrete Structures—Test Methods—Compatibility on Wet Concrete; European Committee for Standarization: Brussels, Belgium, 2003; Available online: https://shop.bsigroup.com/ProductDetail/?pid=000000000030001938.

| Material | Type | Composition |
|---|---|---|
| Cement | CEM I 42.5 R | 410 kg/m3 |
| Water | Tab water | 184.5 kg/m3 |
| Limestone sand | 0–4 mm | 895 kg/m3 |
| Limestone grit | 4–8 mm | 895 kg/m3 |
| Plasticizer | SP Viscocrete | 0.5 wt.% of cement |
| Material | MgO | SiO2 | CaO | Fe2O3 | Al2O3 | SO3 | LOI |
|---|---|---|---|---|---|---|---|
| Mg(OH)2 | 63.12 | 4.25 | 2.46 | 0.25 | 0.1 | 0.02 | 30.11 |
| Thick Layer | Thin Layer | |||||
|---|---|---|---|---|---|---|
| Coating | fh (MPa) | Type of Failure | fh (MPa) | Type of Failure | ||
| A/B (%) 1 | B (%) 2 | A/B (%) 1 | B (%) 2 | |||
| M1m | 0.6 | 20 | 80 | 0.5 | 30 | 70 |
| M2m | 0.3 | 30 | 70 | 0.3 | 70 | 30 |
| M3m | 0.1 | 100 | 0 | 0.1 | 90 | 10 |
| M1mc | 0.3 | 100 | 0 | 0.3 | 100 | 0 |
| M2mc | 0.1 | 100 | 0 | 0.1 | 95 | 5 |
| M3mc | 0.1 | 100 | 0 | 0.1 | 95 | 5 |
| 7% | 17% | 35% | ||||
|---|---|---|---|---|---|---|
| Thin Layer | Thick Layer | Thin Layer | Thick Layer | Thin Layer | Thick Layer | |
| M1m | +21 | +40 | +67 | +133 | +67 | +167 |
| M2m | +7 | +20 | +67 | +133 | +67 | +133 |
| M3m | +18 | +33 | +67 | +100 | +33 | +100 |
| M1mc | +14 | +40 | +67 | +133 | +67 | +133 |
| M2mc | +14 | +33 | +67 | +150 | +67 | +133 |
| M3mc | +7 | +47 | +67 | +100 | +67 | +100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merachtsaki, D.; Tsardaka, E.-C.; Tsampali, E.; Simeonidis, K.; Anastasiou, E.; Yiannoulakis, H.; Zouboulis, A. Study of Corrosion Protection of Concrete in Sewage Systems with Magnesium Hydroxide Coatings. Environ. Sci. Proc. 2020, 2, 27. https://doi.org/10.3390/environsciproc2020002027
Merachtsaki D, Tsardaka E-C, Tsampali E, Simeonidis K, Anastasiou E, Yiannoulakis H, Zouboulis A. Study of Corrosion Protection of Concrete in Sewage Systems with Magnesium Hydroxide Coatings. Environmental Sciences Proceedings. 2020; 2(1):27. https://doi.org/10.3390/environsciproc2020002027
Chicago/Turabian StyleMerachtsaki, Domna, Eirini-Chrysanthi Tsardaka, Evangelia Tsampali, Konstantinos Simeonidis, Eleftherios Anastasiou, Haris Yiannoulakis, and Anastasios Zouboulis. 2020. "Study of Corrosion Protection of Concrete in Sewage Systems with Magnesium Hydroxide Coatings" Environmental Sciences Proceedings 2, no. 1: 27. https://doi.org/10.3390/environsciproc2020002027
APA StyleMerachtsaki, D., Tsardaka, E.-C., Tsampali, E., Simeonidis, K., Anastasiou, E., Yiannoulakis, H., & Zouboulis, A. (2020). Study of Corrosion Protection of Concrete in Sewage Systems with Magnesium Hydroxide Coatings. Environmental Sciences Proceedings, 2(1), 27. https://doi.org/10.3390/environsciproc2020002027









