Micromixing Nanoparticles and Contaminated Water Under Different Velocities for Optimum Heavy Metal Ions Adsorption †
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sofos, F.; Karakasidis, T.E.; Spetsiotis, D. Molecular dynamics simulations of ion separation in nano-channel water flows using an electric field. Mol. Simul. 2019, 45, 1395–1402. [Google Scholar] [CrossRef]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Babel, S.; Kurniawan, T.A. Low-Cost adsorbents for heavy metals uptake from contaminated water: A review. J. Hazard. Mater. 2003, 97, 219–243. [Google Scholar] [CrossRef]
- Karvelas, E.; Liosis, C.; Karakasidis, T.; Sarris, I. Mixing of particles in micromixers under different angles and velocities of the incoming water. Proceedings 2018, 2, 577. [Google Scholar] [CrossRef]
- Bönnemann, H.; Nagabhushana, K.S.; Richards, R.M. Colloidal nanoparticles stabilized by surfactants or organo-aluminum derivatives: Preparation and use as catalyst precursors. In Nanoparticles and Catalysis; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar] [CrossRef]
- Beni, A.A.; Esmaeili, A. Biosorption, an efficient method for removing heavy metals from industrial effluents: A review. Environ. Technol. Innov. 2020, 17, 100503. [Google Scholar] [CrossRef]
- Podstawczyk, D.; Witek-Krowiak, A.; Dawiec, A.; Bhatnagar, A. Biosorption of copper(II) ions by flax meal: empirical modeling and process optimization by response surface methodology (RSM) and artificial neural network (ANN) simulation. Ecol. Eng. 2015, 83, 364–379. [Google Scholar] [CrossRef]
- Bronstein, L.M.; Matveeva, V.G.; Sulman, E.M. Nanoparticulate catalysts based on nanostructured polymers. In NNanoparticles and Catalysis; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar] [CrossRef]
- Somorjai, G.A.; Contreras, A.M.; Montano, M.; Rioux, R.M. Clusters, surfaces, and catalysis. Procceedings 2006, 103, 10577–10583. [Google Scholar] [CrossRef] [PubMed]
- Astruc, D. Transitio-Metal nanoparticles in catalysis : From historical background to the stat-of-the art. In Nanoparticles and Catalysis; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar]
- Chang, Y.-C.; Chen, D.-H. Preparation and adsorption properties of monodisperse chitosan-bound fe3o4 magnetic nanoparticles for removal of Cu(II) Ions. J. Colloid Interface Sci. 2005, 283, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Ain, Q.-U.; Farooq, M.U.; Jalees, M.I. Application of magnetic graphene oxide for water purification: heavy metals removal and disinfection. J. Water Process Eng. 2020, 33, 101044. [Google Scholar] [CrossRef]
- Bartzis, V.; Sarris, I.E. A Theoretical model for salt ion drift due to electric field suitable to seawater desalination. Desalination 2020, 473, 114163. [Google Scholar] [CrossRef]
- Asghari, E.; Moosavi, A.; Hannani, S.K. Simulation of water purification using magnetically ultra-responsive micro- and nanoscavengers. J. Water Process Eng. 2018, 24, 63–73. [Google Scholar] [CrossRef]
- Liosis, C.; Karvelas, E.G.; Karakasidis, T.; Sarris, I.E. Numerical study of magnetic particles mixing in waste water under an external magnetic field. J. Water Supply Res. Technol. 2020, 1–10. [Google Scholar] [CrossRef]
- Karvelas, E.; Liosis, C.; Benos, L.; Karakasidis, T.; Sarris, I. Micromixing efficiency of particles in heavy metal removal processes under various inlet conditions. Water 2019, 11, 1135. [Google Scholar] [CrossRef]
- Karvelas, E.G.; Lampropoulos, N.K.; Sarris, I.E. A numerical model for aggregations formation and magnetic driving of spherical particles based on OpenFOAM®. Comput. Methods Programs Biomed. 2017, 142, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Sofos, F.; Liakopoulos, A.; Karakasidis, T.E. Particle-Based modeling and meshless simulation of flows with smoothed particle hydrodynamics. Glob. NEST J. 2019, 21, 513–518. [Google Scholar] [CrossRef]
- Weller, H.G.; Tabor, G.; Jasak, H.; Fureby, C. A tensorial approach to computational continuum mechanics using object-oriented techniques. Comput. Phys. 1998, 12, 620. [Google Scholar] [CrossRef]
- Ramesha, D.K.; Anvekar, A.; Raj, A.; Vighnesh, J.; Tripathi, S. A DSMC analysis of gas flow in micro channels using OpenFOAM. In Proceedings of the International Conference on Advances in Mechanical Engineering Sciences (ICAMES-17), Mandya, Karnataka, 21–22 April 2017. [Google Scholar]
- Tijskens, E.; Ramon, H.; Baerdemaeker, J.D. Discrete element modelling for process simulation in agriculture. J. Sound Vib. 2003, 266, 493–514. [Google Scholar] [CrossRef]
- Cao, Q.; Han, X.; Li, L. An active microfluidic mixer utilizing a hybrid gradient magnetic field. Int. J. Appl. Electromagn. Mech. 2015, 47, 583–592. [Google Scholar] [CrossRef]
- Yoo, J.; Kim, H.S.; Park, S.Y.; Kwon, S.; Lee, J.; Koo, J.; Seo, Y.S. Instantaneous integration of magnetite nanoparticles on graphene oxide assisted by ultrasound for efficient heavy metal ion retrieval. Ultrason. Sonochem. 2020, 64, 104962. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ni, S.; Wang, X.; Zhang, W.; Lagerquist, L.; Qin, M.; Willför, S.; Xu, C.; Fatehi, P. Ultrafast adsorption of heavy metal ions onto functionalized lignin-based hybrid magnetic nanoparticles. Chem. Eng. J. 2019, 372, 82–91. [Google Scholar] [CrossRef]



| Heavy Metal | MCL (mg/L) |
|---|---|
| Arsenic (As) | 0.05 |
| Cadmium (Cd) | 0.01 |
| Chromium (Cr) | 0.05 |
| Copper (Cu) | 0.25 |
| Nickel (Ni) | 0.20 |
| Zinc (Zn) | 0.80 |
| Lead (Pb) | 0.006 |
| Mercury (Hg) | 0.00003 |
| Simulation Parameters | ||
|---|---|---|
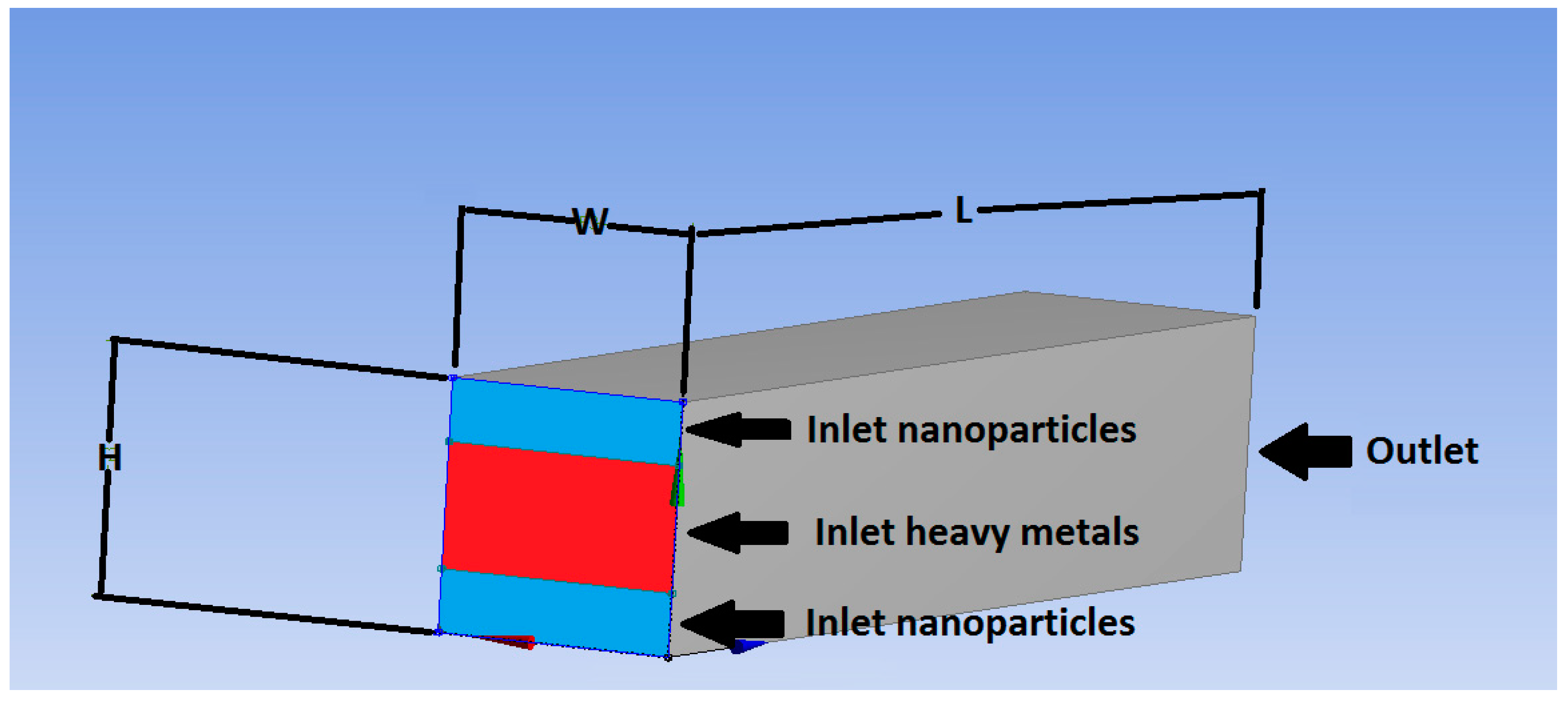
| Dimensions of the micromixer geometry | Length (L): 5 × 10−4 m, Height (H): 1 × 10−4 m, Width (W): 1 × 10−4 m | |
| Diameter of nanoparticles | 40 nm | |
| Diameter of heavy metals | 0.4 nm | |
| Nanoparticles per second | 3000 | |
| Heavy metals per second | 1500 | |
| Permanent magnetic field | 10 T | |
| Gradient magnetic field | 10 T/m | |
| Frequency | 0.1, 1, 5 (Hz) | |
| Boundary conditions | ||
| Boundary | Velocity (U) (m/s) | Pressure (p) (pa) |
| Contaminated water-heavy metals (Vc) | 0.00005, 0.00001, 0.000005, 0.0000025 | zero gradient |
| Nanoparticles (Vp) | 0.00005 | zero gradient |
| Outlet | zero gradient | 0 |
| Walls | 0 | zero gradient |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karvelas, E.; Liosis, C.; Karakasidis, T.; Sarris, I. Micromixing Nanoparticles and Contaminated Water Under Different Velocities for Optimum Heavy Metal Ions Adsorption. Environ. Sci. Proc. 2020, 2, 65. https://doi.org/10.3390/environsciproc2020002065
Karvelas E, Liosis C, Karakasidis T, Sarris I. Micromixing Nanoparticles and Contaminated Water Under Different Velocities for Optimum Heavy Metal Ions Adsorption. Environmental Sciences Proceedings. 2020; 2(1):65. https://doi.org/10.3390/environsciproc2020002065
Chicago/Turabian StyleKarvelas, Evangelos, Christos Liosis, Theodoros Karakasidis, and Ioannis Sarris. 2020. "Micromixing Nanoparticles and Contaminated Water Under Different Velocities for Optimum Heavy Metal Ions Adsorption" Environmental Sciences Proceedings 2, no. 1: 65. https://doi.org/10.3390/environsciproc2020002065
APA StyleKarvelas, E., Liosis, C., Karakasidis, T., & Sarris, I. (2020). Micromixing Nanoparticles and Contaminated Water Under Different Velocities for Optimum Heavy Metal Ions Adsorption. Environmental Sciences Proceedings, 2(1), 65. https://doi.org/10.3390/environsciproc2020002065








