In Situ Biogeochemical Barriers for Contaminated Groundwater Treatment near Uranium Sludge Storages †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Objects
2.2. Analysis Methods
3. Results and Discussion
3.1. Biological Purification of Polluted Water
3.2. Future Work on the Formation of Barriers in Situ
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gaskova, O.L.; Boguslavsky, A.E.; Shemelina, O.V. Uranium release from contaminated sludge materials and uptake by subsurface sediments: Experimental study and thermodynamic modeling. Appl. Geochem. 2015, 55, 152–159. [Google Scholar] [CrossRef]
- Vishnyakova, A.; Popova, N.; Artemiev, G.; Botchkova, E.; Litti, Y.; Safonov, A. Effect of Mineral Carriers on Biofilm Formation and Nitrogen Removal Activity by an Indigenous Anammox Community from Cold Groundwater Ecosystem Alone and Bioaugmented with Biomass from a “Warm” Anammox Reactor. Biology 2022, 11, 1421. [Google Scholar] [CrossRef] [PubMed]
- Safonov, A.V.; Babich, T.L.; Sokolova, D.S.; Grousdev, D.S.; Tourova, T.P.; Poltaraus, A.B.; Zakharova, E.V.; Merkel, A.Y.; Novikov, A.P.; Nazina, T.N. Microbial Community and in situ Bioremediation of Groundwater by Nitrate Removal in the Zone of a Radioactive Waste Surface Repository. Front. Microbiol. 2018, 9, 1985. [Google Scholar] [PubMed]
- Safonov, A.; Popova, N.; Boldyrev, K.; Lavrinovich, E.; Boeva, N.; Artemiev, G.; Kuzovkina, E.; Emelyanov, A.; Myasnikov, I.; Zakharova, E.; et al. The microbial impact on U, Pu, Np, and Am immobilization on aquifer sandy rocks, collected at the deep LRW injection site. J. Geochem. Explor. 2022, 240, 107052. [Google Scholar] [CrossRef]
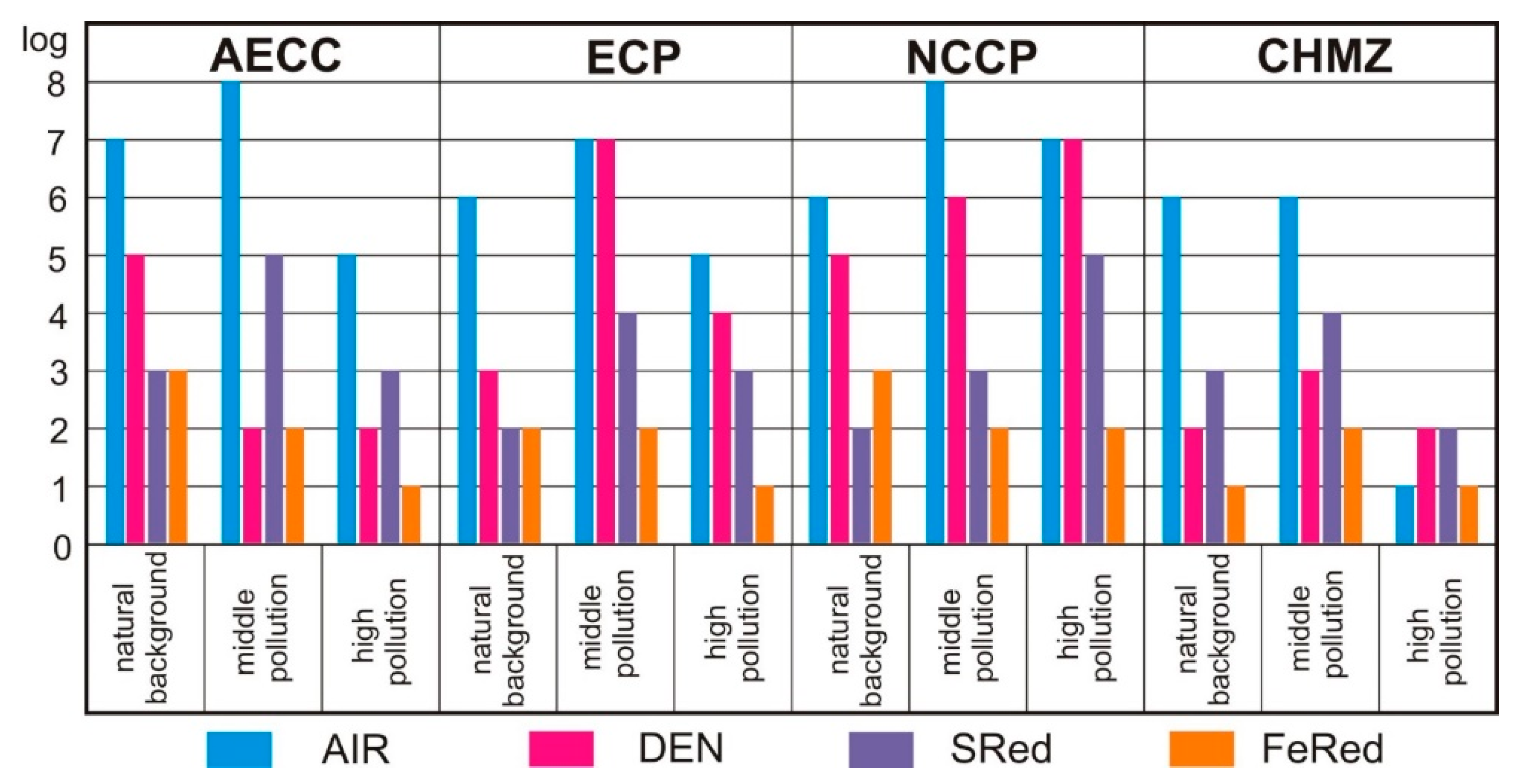
| AECC | ECP | NZHK | CHMZ | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Natural | Middle | Polluted | Natural | Middle | Polluted | Natural | Middle | Polluted | Natural | Middle | Polluted | ||
| Eh | mV | 115 | 136 | 34 | 166 | 90 | 94 | −4 | −29 | 57 | −70 | 162 | 195 |
| pH | 7.39 | 7.10 | 9.78 | 7.35 | 6.70 | 7.50 | 7.01 | 6.51 | 7.40 | 7.20 | 7.80 | 6.50 | |
| CO3− + HCO3− | ppm | 96 | 89 | 412 | 267 | 215 | 204 | 393 | 151 | 195 | 54 | 212 | 319 |
| SO42− | 27 | 1620 | 3720 | 0.55 | 143 | 800 | 24.4 | 1920 | 590 | 21.4 | 1585 | 780 | |
| Cl− | 6.45 | 49 | 350 | 1.61 | 65 | 17 | 21 | 750 | 2984 | 10.9 | 1131 | 2260 | |
| NH4+ | 0.28 | 0.20 | 103 | 9.1 | 16.5 | 68 | 1.0 | 1.1 | 120 | 7.8 | 88 | 292 | |
| NO3− | 0.98 | 45.4 | 2440 | 0.80 | 2330 | 11,500 | 5.9 | 900 | 4740 | 11.5 | 3460 | 7100 | |
| Ca2+ | 33.9 | 299.1 | 114.6 | 77.8 | 602 | 5340 | 103 | 556 | 804 | 132 | 1113 | 2940 | |
| Na | 5.25 | 94.22 | 1519 | 11.74 | 70.73 | 127 | 12.40 | 563 | 2013 | 100 | 1058 | 1549 | |
| Mg | 18 | 120 | 5.0 | 25 | 60 | 120 | 35 | 160 | 72 | 31 | 29 | 76 | |
| K | 0.72 | 3.09 | 139 | 1.99 | 5.00 | 11.0 | 1.83 | 8.92 | 91 | 16.5 | 234 | 47 | |
| Si | 7.54 | 7.11 | 1.27 | 5.73 | 5.47 | 7.00 | 6.74 | 1.19 | 4.87 | 3.20 | 12.4 | 19.7 | |
| Al | 0.04 | 0.08 | 0.13 | 0.07 | 0.09 | 0.09 | 0.11 | 0.08 | 0.13 | 0.14 | 2.4 | 3.1 | |
| Fe | 13.9 | 1.66 | 3.33 | 1.59 | 2.03 | 15.4 | 5.26 | 3.46 | 2.65 | 1.40 | 5.10 | 67 | |
| Mn | 0.41 | 0.07 | 0.81 | 1.1 | 0.73 | 2.2 | 1.3 | 2.1 | 2.5 | 0.83 | 1.0 | 2.4 | |
| P | 0.20 | 0.31 | 1.16 | 0.40 | 1.29 | 10.0 | 0.53 | 0.47 | 0.07 | 0.01 | 0.01 | 0.39 | |
| U | ppb | 0.09 | 0.24 | 0.26 | 0.23 | 3.0 | 5.6 | 2.0 | 0.86 | 3422 | 0.01 | 1.90 | 4930 |
| The Media | Duration of NO3− Decomposition, Days | Rapidity of NO3− Decomposition, ppb/Day | Duration of SO42− Decomposition, Days | Rapidity of SO42− Decomposition, ppb/Day | |
|---|---|---|---|---|---|
| AECC | natural | 0 | 0.08 | 12 | 27 * |
| middle | 5 | 1.5 | 30 | 324 | |
| polluted | 20 | 32.5 | 75 | 186 | |
| ECP | natural | 0 | 0.8 * | 0 | 0.55 * |
| middle | 25 | 58.2 | 40 | 5.7 | |
| polluted | 180 | 54.8 | 210 | 4.4 | |
| NZHK | natural | 0 | 0.8 | 7 | 25 * |
| middle | 7 | 20 | 45 | 274 | |
| polluted | 75 | 36.4 | 130 | 7.9 | |
| CHMZ | natural | 0 | 0.8 | 14 | 21 * |
| middle | 45 | 38.4 | 90 | 1585 | |
| polluted | 90 | 54.6 | 130 | 780 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boguslavsky, A.; Safonov, A.; Shvartseva, O. In Situ Biogeochemical Barriers for Contaminated Groundwater Treatment near Uranium Sludge Storages. Environ. Sci. Proc. 2023, 25, 66. https://doi.org/10.3390/ECWS-7-14244
Boguslavsky A, Safonov A, Shvartseva O. In Situ Biogeochemical Barriers for Contaminated Groundwater Treatment near Uranium Sludge Storages. Environmental Sciences Proceedings. 2023; 25(1):66. https://doi.org/10.3390/ECWS-7-14244
Chicago/Turabian StyleBoguslavsky, Anatoly, Alexey Safonov, and Olga Shvartseva. 2023. "In Situ Biogeochemical Barriers for Contaminated Groundwater Treatment near Uranium Sludge Storages" Environmental Sciences Proceedings 25, no. 1: 66. https://doi.org/10.3390/ECWS-7-14244
APA StyleBoguslavsky, A., Safonov, A., & Shvartseva, O. (2023). In Situ Biogeochemical Barriers for Contaminated Groundwater Treatment near Uranium Sludge Storages. Environmental Sciences Proceedings, 25(1), 66. https://doi.org/10.3390/ECWS-7-14244







