Activated Carbon, CNTs and GO Based Polymeric Nanocomposites Membranes for Textile Wastewater Treatment: Preparation, Performance, and Fouling Control †
Abstract
:1. Introduction
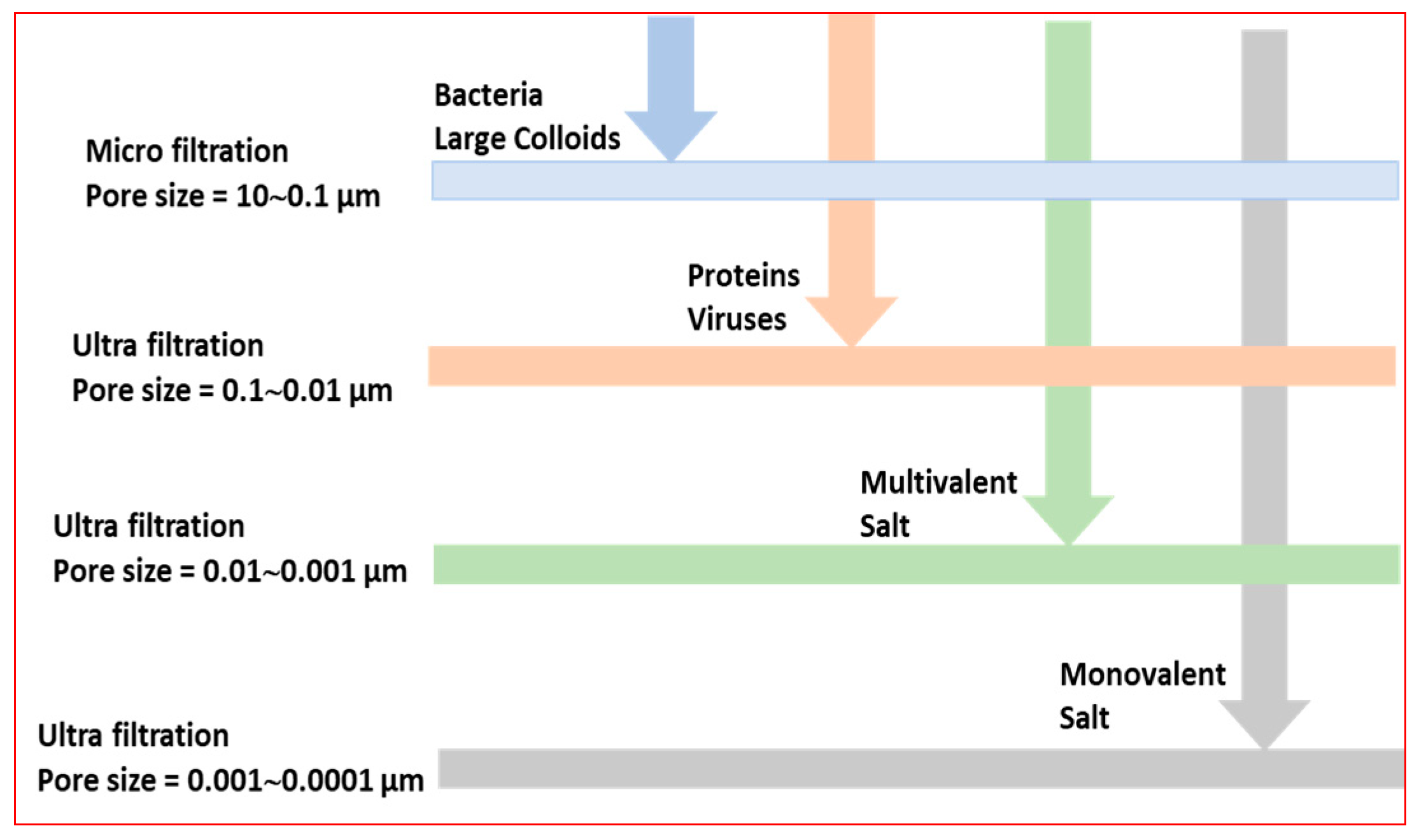
1.1. Current Water Treatment Capabilities
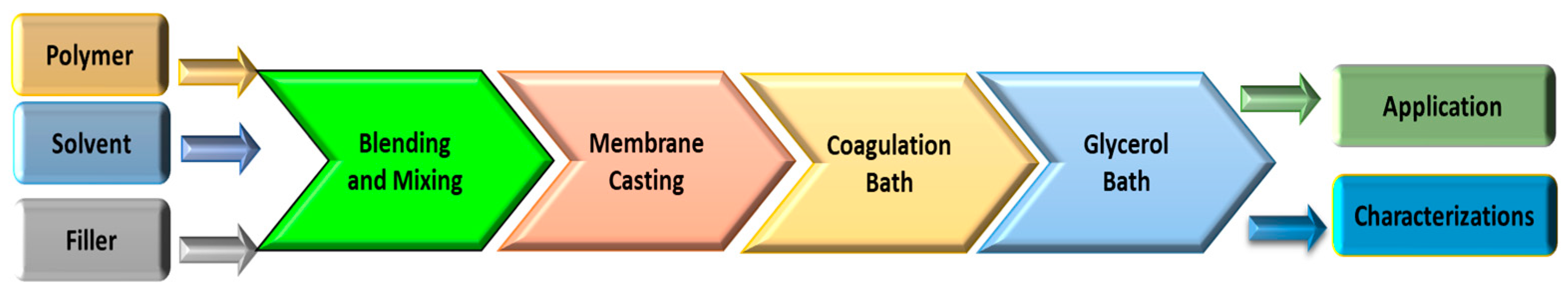
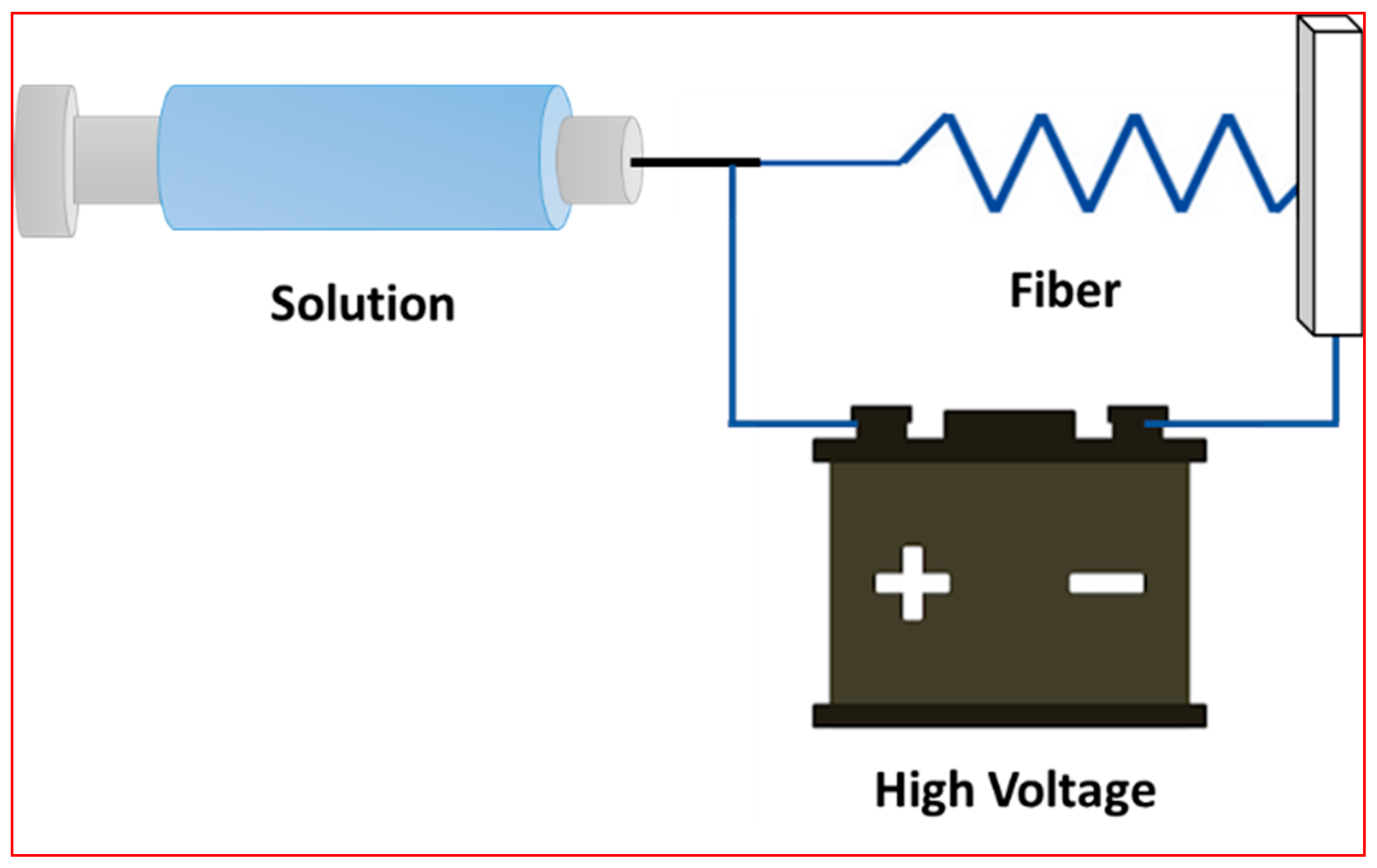
1.2. Membrane Fabrication Methods
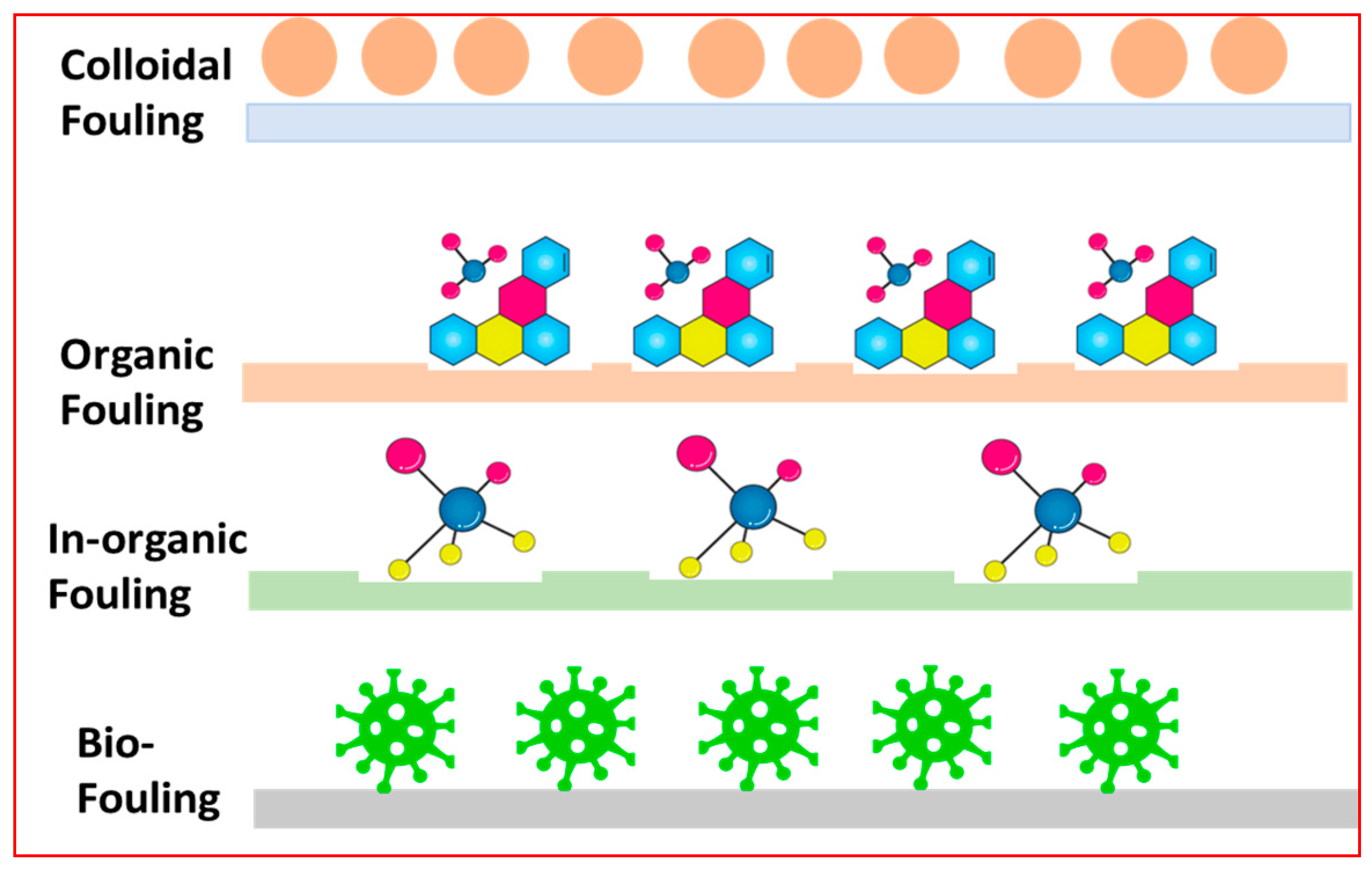
1.3. Membrane Fouling
2. Carbon-Based Materials for Filtration
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mekonnen, M.; Hoekstra, A.Y. Sustainability: Four billion people facing severe water scarcity. Sci. Adv. 2016, 2, e1500323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brusseau, M.L.; Ramirez-Andreotta, M.; Pepper, I.L.; Maximillian, J. Environmental Impacts on Human Health and Well-Being, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128147191. [Google Scholar]
- Tavangar, T.; Jalali, K.; Alaei Shahmirzadi, M.A.; Karimi, M. Toward real textile wastewater treatment: Membrane fouling control and effective fractionation of dyes/inorganic salts using a hybrid electrocoagulation—Nanofiltration process. Sep. Purif. Technol. 2019, 216, 115–125. [Google Scholar] [CrossRef]
- Tahri, N.; Masmoudi, G.; Ellouze, E.; Jrad, A.; Drogui, P.; Ben Amar, R. Coupling microfiltration and nanofiltration processes for the treatment at source of dyeing-containing effluent. J. Clean. Prod. 2012, 33, 226–235. [Google Scholar] [CrossRef]
- Keskin, B.; Ersahin, M.E.; Ozgun, H.; Koyuncu, I. Pilot and full-scale applications of membrane processes for textile wastewater treatment: A critical review. J. Water Process Eng. 2021, 42, 102172. [Google Scholar] [CrossRef]
- Keskin, B.; Ağtaş, M.; Ormancı-Acar, T.; Türken, T.; Imer, D.Y.; Ünal, S.; Menceloğlu, Y.Z.; Uçar-Demir, T.; Koyuncu, I. Halloysite nanotube blended nanocomposite ultrafiltration membranes for reactive dye removal. Water Sci. Technol. 2021, 83, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Obotey Ezugbe, E.; Rathilal, S. Membrane Technologies in Wastewater Treatment: A Review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, G.Z.; Mitropoulos, A.C. Polymeric Materials for Water and Wastewater Management. Polymers 2021, 13, 168. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.W. Membrane Technology Enhanced Reader, 3rd ed.; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Kochkodan, V.; Johnson, D.J.; Hilal, N. Polymeric membranes: Surface modification for minimizing (bio)colloidal fouling. Adv. Colloid Interface Sci. 2014, 206, 116–140. [Google Scholar] [CrossRef] [PubMed]
- Strathmann, H. The Formation Mechanism of Phase Inversion Membranes. Desalination 1977, 21, 241–255. [Google Scholar] [CrossRef]
- Yu, F.; Shi, H.; Shi, J.; Teng, K.; Xu, Z.; Qian, X. High-performance forward osmosis membrane with ultra-fast water transport channel and ultra-thin polyamide layer. J. Memb. Sci. 2020, 616, 118611. [Google Scholar] [CrossRef]
- Kochkodan, V.; Hilal, N. A comprehensive review on surface modified polymer membranes for biofouling mitigation. Desalination 2015, 356, 187–207. [Google Scholar] [CrossRef]
- Francois, P.; Andreia, F.; Menachem, E. Antimicrobial Properties of Graphene Oxide Nanosheets Why Size Matters. ACS Nano 2015, 9, 7226–7236. [Google Scholar] [CrossRef]
- Li, Z.; Hanafy, H.; Zhang, L.; Sellaoui, L.; Schadeck Netto, M.; Oliveira, M.L.S.; Seliem, M.K.; Luiz Dotto, G.; Bonilla-Petriciolet, A.; Li, Q. Adsorption of congo red and methylene blue dyes on an ashitaba waste and a walnut shell-based activated carbon from aqueous solutions: Experiments, characterization and physical interpretations. Chem. Eng. J. 2020, 388, 124263. [Google Scholar] [CrossRef]
- Manoukian, M.; Fashandi, H.; Tavakol, H. Polysulfone-highly uniform activated carbon sphere mixed-matrix membrane intended for efficient purification of dye wastewater. Mater. Res. Express 2019, 6, 055313. [Google Scholar] [CrossRef]
- Mohammed, M.I.; Abdul Razak, A.A.; Hussein Al-Timimi, D.A. Modified multiwalled carbon nanotubes for treatment of some organic dyes in wastewater. Adv. Mater. Sci. Eng. 2014, 2014, 201052. [Google Scholar] [CrossRef] [Green Version]
- Tiraferri, A.; Vecitis, C.D.; Elimelech, M. Covalent binding of single-walled carbon nanotubes to polyamide membranes for antimicrobial surface properties. ACS Appl. Mater. Interfaces 2011, 3, 2869–2877. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.X.; Gao, Y.; Yang, C.; Wang, Z.Q.; Xue, G. Facile and controllable assembly of multiwalled carbon nanotubes on polystyrene microspheres. Chin. J. Polym. Sci. Engl. Ed. 2014, 32, 711–717. [Google Scholar] [CrossRef]
- Leaper, S.; Abdel-Karim, A.; Faki, B.; Luque-Alled, J.M.; Alberto, M.; Vijayaraghavan, A.; Holmes, S.M.; Szekely, G.; Badawy, M.I.; Shokri, N.; et al. Flux-enhanced PVDF mixed matrix membranes incorporating APTS-functionalized graphene oxide for membrane distillation. J. Memb. Sci. 2018, 554, 309–323. [Google Scholar] [CrossRef]
- Leaper, S.; Abdel-Karim, A.; Gorgojo, P. The use of carbon nanomaterials in membrane distillation membranes: A review. Front. Chem. Sci. Eng. 2021, 15, 755–774. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, I.A.; Ahmad, N.M. Activated Carbon, CNTs and GO Based Polymeric Nanocomposites Membranes for Textile Wastewater Treatment: Preparation, Performance, and Fouling Control. Environ. Sci. Proc. 2023, 25, 77. https://doi.org/10.3390/ECWS-7-14307
Khan IA, Ahmad NM. Activated Carbon, CNTs and GO Based Polymeric Nanocomposites Membranes for Textile Wastewater Treatment: Preparation, Performance, and Fouling Control. Environmental Sciences Proceedings. 2023; 25(1):77. https://doi.org/10.3390/ECWS-7-14307
Chicago/Turabian StyleKhan, Imran A., and Nasir M. Ahmad. 2023. "Activated Carbon, CNTs and GO Based Polymeric Nanocomposites Membranes for Textile Wastewater Treatment: Preparation, Performance, and Fouling Control" Environmental Sciences Proceedings 25, no. 1: 77. https://doi.org/10.3390/ECWS-7-14307
APA StyleKhan, I. A., & Ahmad, N. M. (2023). Activated Carbon, CNTs and GO Based Polymeric Nanocomposites Membranes for Textile Wastewater Treatment: Preparation, Performance, and Fouling Control. Environmental Sciences Proceedings, 25(1), 77. https://doi.org/10.3390/ECWS-7-14307




