The Implementation of Laboratory Information Management System in Multi-Site Genetics Study in Africa: The Challenges and Up-Scaling Opportunities
Abstract
:1. Introduction
2. Materials and Methods
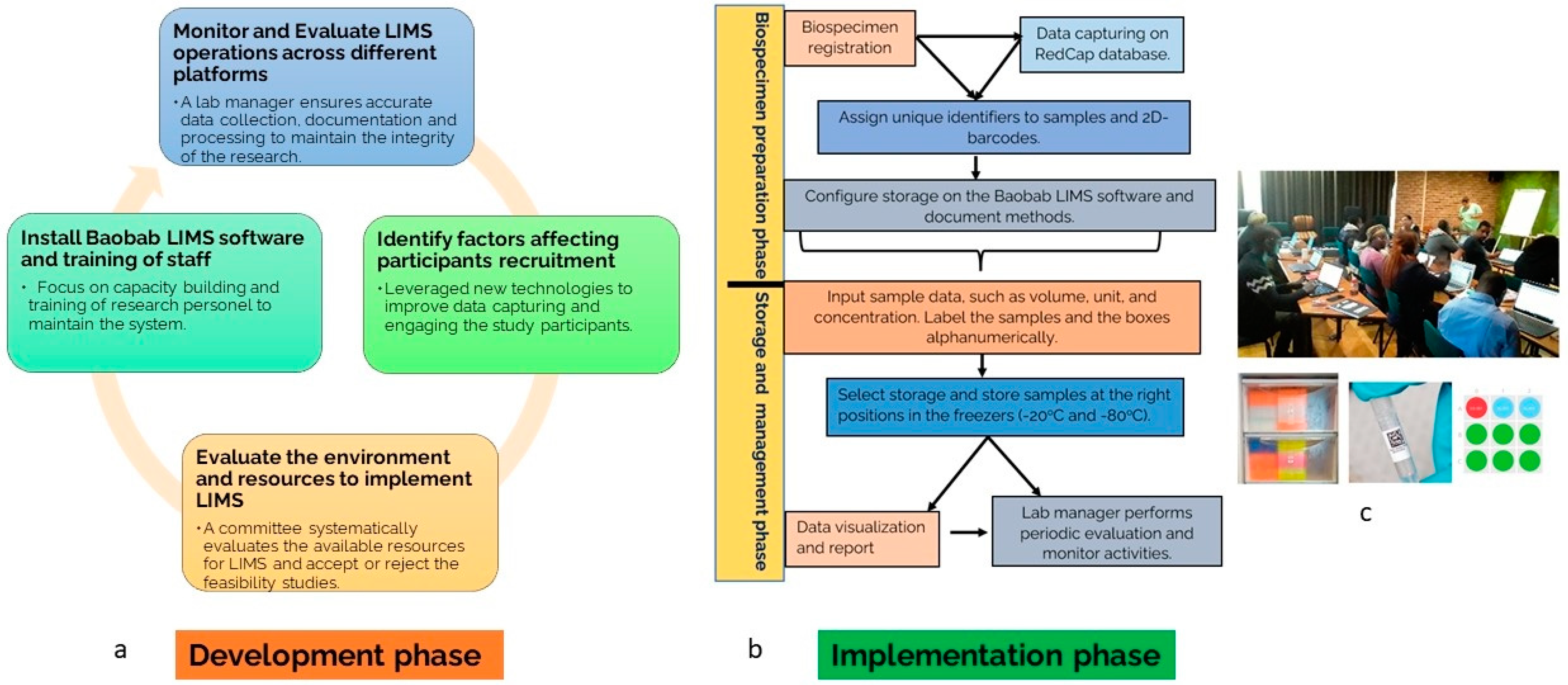
2.1. LIMS Development Phase
2.2. LIMS Implementation Phase
Biospecimen Collection and Data Capturing
2.3. LIMS Data Capture and Management
2.4. Biospecimen Labeling and Barcoding System
2.5. Sample Storage and Tracking
2.6. Biospecimen Quality Control Before Genomics Studies
2.7. Model for LIMS and Artificial Intelligence System
3. Results
- Family members on the recruitment list may be living in geographically different locations;
- A need for translators to inform participants in their local language and vocabulary;
- Difficulty collecting samples, such as blood, from small children.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skobelev, D.O.; Zaytseva, T.M.; Kozlov, A.D.; Perepelitsa, V.L.; Makarova, A.S. Laboratory information management systems in the work of the analytic laboratory. Meas. Tech. 2011, 53, 1182–1189. [Google Scholar] [CrossRef]
- Isfahani, S.S.; Khajouei, R.; Jahanbakhsh, M.; Mirmohamadi, M. The evaluation of hospital laboratory information management systems based on the standards of the American National Standard Institute. J. Educ. Health Promot. 2014, 3, 61. [Google Scholar]
- Laboratory Information Management System (LIMS) Market-Global Forecast to 2025|MarketsandMarkets. Available online: https://www.marketsandmarkets.com/Market-Reports/laboratory-information-management-systems-market-250610373.html?gclid=Cj0KCQiAyJOBBhDCARIsAJG2h5edRqsMsUGxquSITQNHcu-XM3kcdnx6soJoCBBeK-q0tG6TQLsjvqsaAptZEALw_wcB. (accessed on 18 June 2022).
- Colangeli, P. Laboratory information management system: An example of international cooperation in Namibia. Vet. Ital. 2012, 48, 241–251. [Google Scholar] [PubMed]
- Nyasulu, P.S.; Paszko, C.; Mbelle, N.A. Narrative Review of the Laboratory Information System and Its Role in Antimicrobial Resistance Surveillance in South Africa. Adv. Microbiol. 2014, 4, 692–696. [Google Scholar] [CrossRef] [Green Version]
- Chang, A.Y.; Skirbekk, V.F.; Tyrovolas, S.; Kassebaum, N.J.; Dieleman, J.L. Measuring population ageing: An analysis of the Global Burden of Disease Study 2017. Lancet Public Health 2019, 4, e159–e167. [Google Scholar] [CrossRef] [Green Version]
- 2012 best practices for repositories collection, storage, retrieval, and distribution of biological materials for research international society for biological and environmental repositories. Biopreservation Biobanking 2012, 10, 79–161. [CrossRef] [Green Version]
- Mitchell, D. Biobanking from the patient perspective. Res. Involv. Engagem. 2015, 1, 4. [Google Scholar] [CrossRef] [Green Version]
- Riegman, P.H.J.; Morente, M.M.; Betsou, F.; de Blasio, P.; Geary, P. Biobanking for better healthcare. Mol. Oncol. 2008, 2, 213–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, W.C.; Sexton, K.C.; Grizzle, W.E. Organizational issues in providing high-quality human tissues and clinical information for the support of biomedical research. Methods Mol. Biol. Clifton NJ 2010, 576, 1–30. [Google Scholar]
- Engel, K.B.; Vaught, J.; Moore, H.M. National Cancer Institute Biospecimen Evidence-Based Practices: A Novel Approach to Pre-analytical Standardization. Biopreservation Biobanking 2014, 12, 148–150. [Google Scholar] [CrossRef] [Green Version]
- Malentacchi, F. Influence of pre-analytical procedures on genomic DNA integrity in blood samples: The SPIDIA experience. Clin. Chim. Acta Int. J. Clin. Chem. 2015, 440, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Katsanis, S.H.; Katsanis, N. Molecular genetic testing and the future of clinical genomics. Nat. Rev. Genet. 2013, 14, 415–426. [Google Scholar] [CrossRef] [PubMed]
- McGuire, A.L. The road ahead in genetics and genomics. Nat. Rev. Genet. 2020, 21, 581–596. [Google Scholar] [CrossRef]
- Janzen, W. Establishing and Maintaining a Robust Sample Management System. SLAS Technol. Transl. Life Sci. Innov. 2019, 24, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.W. A High-Density Admixture Map for Disease Gene Discovery in African Americans. Am. J. Hum. Genet. 2004, 74, 1001–1013. [Google Scholar] [CrossRef] [Green Version]
- Shriner, D. Overview of Admixture Mapping. Curr. Protoc. Hum. Genet. 2013, 1, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Halder, I.; Shriver, M.D. Measuring and using admixture to study the genetics of complex diseases. Hum. Genom. 2003, 1, 52–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wonkam Tingang, E. Hearing Impairment Overview in Africa: The Case of Cameroon. Genes 2020, 11, 233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neumeister, V.M. A tissue quality index: An intrinsic control for measurement of effects of preanalytical variables on FFPE tissue. Lab. Investig. J. Tech. Methods Pathol. 2014, 94, 467–474. [Google Scholar] [CrossRef] [Green Version]
- Poste, G. Leveling the playing field: Bringing development of biomarkers and molecular diagnostics up to the standards for drug development. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012, 18, 1515–1523. [Google Scholar] [CrossRef] [Green Version]
- Portier, B.P. Delay to formalin fixation ‘cold ischemia time’: Effect on ERBB2 detection by in-situ hybridization and immunohistochemistry. Mod. Pathol. Off. J. U. S. Can. Acad. Pathol. Inc. 2013, 26, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Xie, R. Factors influencing the degradation of archival formalin-fixed paraffin-embedded tissue sections. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2011, 59, 356–365. [Google Scholar] [CrossRef]
- Morrison, A.P. Reduction in specimen labeling errors after implementation of a positive patient identification system in phlebotomy. Am. J. Clin. Pathol. 2010, 133, 870–877. [Google Scholar] [CrossRef] [PubMed]



| Component | Description |
|---|---|
| 1 Administrator’s role | Provides guidelines for staff and students participating in recruitment as an administrator. |
| 2 Ethics Application | Describes the procedure for ethics application to conduct research with human biospecimens. |
| 3 Informed Consent | Describes the procedure to create informed consent documents and implementation during recruitment. |
| 4 Completion of Questionnaire | Describes the procedure to complete a recruitment questionnaire during recruitment. |
| 5 Communication with Locations | Describes the procedure for communications with schools, hospitals, communities, and places where participants are to be recruited. |
| 6 Recruitment | Describes the procedure for patient recruitment. |
| 7 Biological Sample Handling | Describes the procedure to handle biological samples during recruitment and storage. |
| 8 Biological Sample Coding | Describes the procedure to code biological samples during recruitment. |
| 9DNA Extraction | Describes laboratory standard operating procedures for extractions. |
| 10 Sample management | Describes the LIMS procedure (sample allocation, 2D-coding, shipment). |
| 11 Data and Database Management | Provides instructions on data management, and to assign managers that will have user rights. |
| 12 Data Recording | Describes the procedure for recording source and Case Record Form (CRF) data for research studies (and associated activities). |
| 13 Data Capturing | Describes the procedure for capturing recruitment data in RedCap. |
| 14 Laboratory inventories | Describes the procedures for capturing finance information, maintaining inventory, and capturing information regarding assets and stationery. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oluwole, O.G.; Oosterwyk, C.; Anderson, D.; Adadey, S.M.; Mnika, K.; Manyisa, N.; Yalcouye, A.; Wonkam, E.T.; Aboagye, E.T.; Dia, Y.; et al. The Implementation of Laboratory Information Management System in Multi-Site Genetics Study in Africa: The Challenges and Up-Scaling Opportunities. J. Mol. Pathol. 2022, 3, 262-272. https://doi.org/10.3390/jmp3040022
Oluwole OG, Oosterwyk C, Anderson D, Adadey SM, Mnika K, Manyisa N, Yalcouye A, Wonkam ET, Aboagye ET, Dia Y, et al. The Implementation of Laboratory Information Management System in Multi-Site Genetics Study in Africa: The Challenges and Up-Scaling Opportunities. Journal of Molecular Pathology. 2022; 3(4):262-272. https://doi.org/10.3390/jmp3040022
Chicago/Turabian StyleOluwole, Oluwafemi Gabriel, Chandre Oosterwyk, Dominique Anderson, Samuel Mawuli Adadey, Khuthala Mnika, Noluthando Manyisa, Abdoulaye Yalcouye, Edmond T. Wonkam, Elvis Twumasi Aboagye, Yacouba Dia, and et al. 2022. "The Implementation of Laboratory Information Management System in Multi-Site Genetics Study in Africa: The Challenges and Up-Scaling Opportunities" Journal of Molecular Pathology 3, no. 4: 262-272. https://doi.org/10.3390/jmp3040022
APA StyleOluwole, O. G., Oosterwyk, C., Anderson, D., Adadey, S. M., Mnika, K., Manyisa, N., Yalcouye, A., Wonkam, E. T., Aboagye, E. T., Dia, Y., Uwibambe, E., Jonas, M., Priestley, R., Popel, K., Manyashe, T., de Cock, C., Nembaware, V., & Wonkam, A. (2022). The Implementation of Laboratory Information Management System in Multi-Site Genetics Study in Africa: The Challenges and Up-Scaling Opportunities. Journal of Molecular Pathology, 3(4), 262-272. https://doi.org/10.3390/jmp3040022






