Abstract
Patients with metastatic breast cancer often have respiratory symptoms due to various causes. Trastuzumab is a drug used in the treatment of HER2/neu over-expressing breast cancer patients. Organising pneumonia is a type of idiopathic interstitial pneumonia that mimics infection or progressive disease and can be difficult to diagnose in the setting of malignancy. Making a correct diagnosis is of extreme importance since any delay in the treatment can result in significant adverse patient outcome. Here, we have described a case of organising pneumonia associated with the use of trastuzumab in metastatic breast cancer patients. On the basis of clinical data, including findings such as a decreased PaO2 level and findings on chest CT scan, these patients were diagnosed with drug-induced organising pneumonia. Although it is a rare adverse event associated with trastuzumab, it may cause rapid deterioration without preceding symptoms; hence, even though it is very rare, with an incidence of less than 2%, it is still crucial to intervene so as to prevent the occurrence of such an unfavourable outcome by means of close observation and early diagnosis along with an early withdrawal of the drug and an immediate commencement of corticosteroid therapy.
1. Background
Organising pneumonia (OP) is a type of idiopathic interstitial pneumonia, which is associated with various causes like infections, connective tissue disorders, haematological malignancies, radiation therapy, organ transplantation, etc. An idiopathic form of OP may also be observed. Although lung structure is retained, it is pathologically distinguished by the presence of Masson bodies, which are characterised by the presence of buds of granulation tissue created by fibrin exudates, fibroblasts, myofibroblasts, and loose connective tissue in distant air spaces [1]. Patients with metastatic breast cancer often have respiratory symptoms with various causes. Trastuzumab is a drug used to treat HER2/neu overexpressing breast cancers. Approximately 15–20% of patients with breast cancer have tumours that overexpress human epidermal growth factor receptor 2 (HER2), which are associated with an aggressive clinical phenotype and poor prognosis. As a result of using trastuzumab to treat metastatic breast cancer, a case of organising pneumonia has been described here [2]. This is very rare, with an incidence of less than 2%. This is a periodic case report, with only three such cases being reported previously. It is not a pilot-based study; similar studies have been conducted in the past.
2. Case Study
A 53-year-old female smoker underwent breast-conservative surgery in November 2021 for invasive ductal carcinoma with ductal carcinoma in situ of the right breast. The patient was initially started on chemotherapy with epirubicin and cyclophosphamide. Four cycles of the same were given. The patient was then taken into therapy with four cycles of paclitaxel. There were no respiratory complaints during this time. As soon as the immunohistochemical staining report was received, the patient was shifted to combination therapy with paclitaxel and trastuzumab.
As far as the case of breast cancer is concerned, the sensitivity of the tumour to oestrogen and progesterone is checked, and depending upon the sensitivity, the treatment modality is modified. In this patient, the immunohistochemical staining of the neoplastic cells was negative for oestrogen and progesterone receptors and was positive for HER2/neu receptors. The human epidermal growth factor receptor 2 (HER2) is an active member of the epidermal growth factor receptor family. Tyrosine residues in the receptors’ cytoplasmic domain are auto-phosphorylated due to receptor dimerisation, which opens up several signalling pathways that promote cell growth and the development of tumours.
As a prognostic and predictive biomarker, HER2 amplification or overexpression is found in roughly 15–30% of breast cancer patients and 10–30% of gastric/gastroesophageal cancer patients. Other malignancies like the ovary, endometrial, bladder, lung, colon, and head and neck have also been reported to overexpress HER2. The family is made up of four main members: HER-1, HER-2, HER-3, and HER-4, also called ErbB1, ErbB2, ErbB3, and ErbB4, respectively [1]. All four HER receptors comprise a cysteine-rich extracellular ligand binding site, a transmembrane lipophilic segment, and an intracellular domain with tyrosine kinase catalytic activity.
However, the outcomes have been dismal in other cancer types. The introduction of HER2-directed therapy has significantly improved the outcome of patients with HER2-positive breast and stomach/gastroesophageal cancers.
The patient was then started on chemotherapy with albumin-bound paclitaxel 100 mg/m2 weekly and trastuzumab 4 mg/kg as the loading dose, followed by 2 mg/kg weekly.
The patient did not experience any adverse drug reactions during the initial three cycles of trastuzumab therapy. There were no significant radiological changes noted during these cycles. After her fourth dose, the patient started experiencing dyspnoea that increased progressively. Initially, she only had difficulty breathing during exertion, which progressed to dyspnoea developing even at rest. She had no complaints of fever or haemoptysis.
The patient was planned for radiotherapy, but due to the dyspnoea, she was postponed and referred to the Respiratory Medicine Department. She visited the department on an outpatient basis. She presented with the chief complaints of weakness, effort dyspnoea, and non-productive cough. Her vitals were stable. On auscultation, coarse crackles were heard at the base of her right lung.
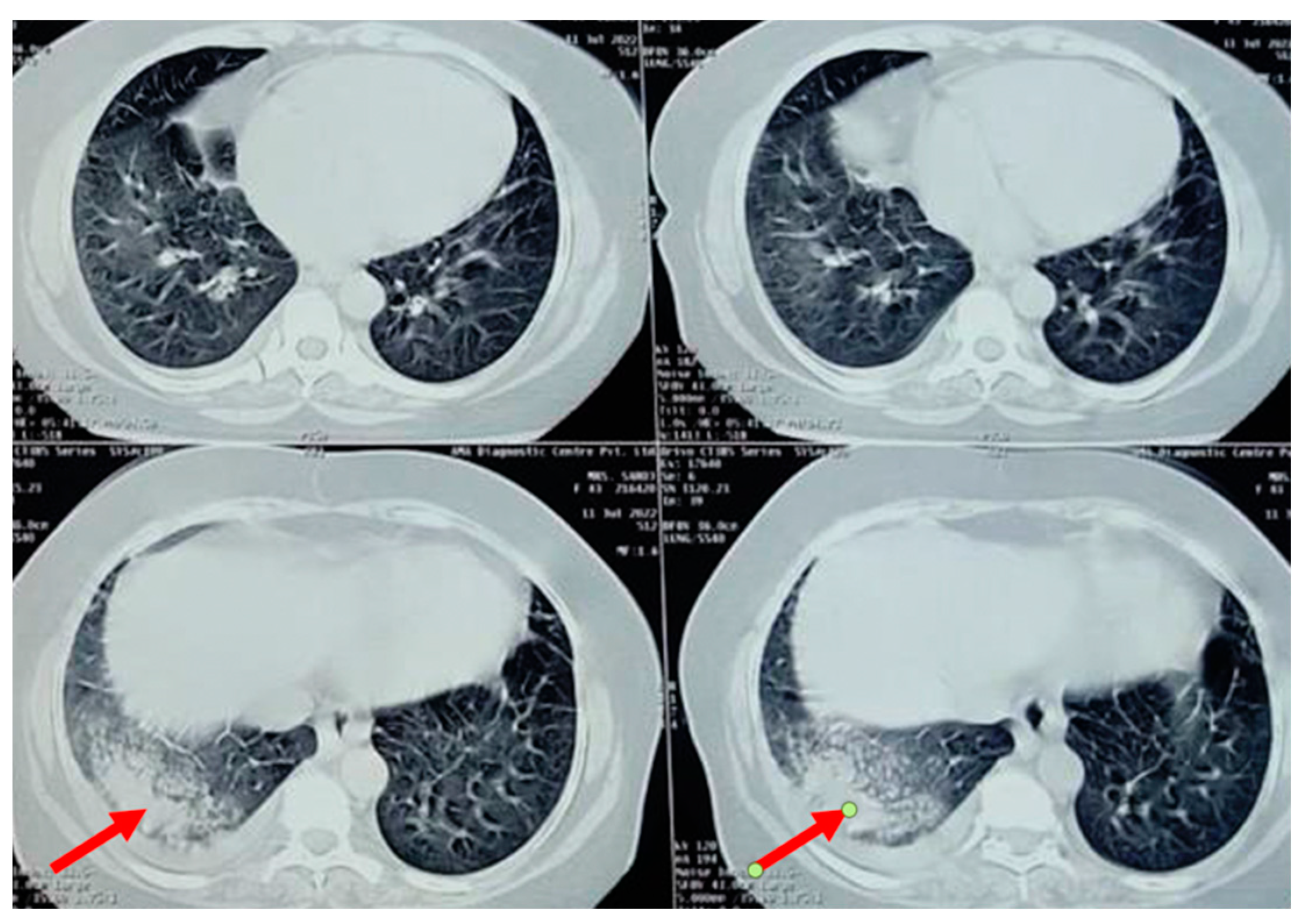
A chest radiograph showed patchy consolidation in the right lower lobe and mild pleural effusion. To obtain a better view of the lesion, a high-resolution computed tomography scan was performed, which showed patchy foci of air space consolidation in the right lower lobe along with pleural effusion (Figure 1).
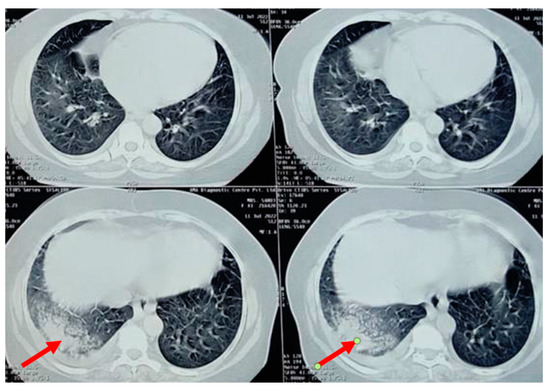
Figure 1.
High-resolution computed tomography scan of the thorax of a 58-year-old female showing right lower lobe consolidation with “ground glass” opacity, indicated by the red arrow (trastuzumab-induced).
The patient was then subjected to pulmonary function tests. The pulmonary function tests revealed a mixed pattern. Force expiratory volume in one second (FEV1) and force vital capacity (FVC) ratio post-bronchodilator were 65%, whereas FVC pre-bronchodilator was 52% and post-bronchodilator was 59%, which are suggestive of obstruction with restriction (mixed pattern). A series of blood investigations were performed. The blood parameters, including D dimer, erythrocyte sedimentation rate (ESR), anti-neutrophil antibody (ANA), and rheumatological profile, were within normal limits.
The patient developed a dry cough after receiving her fourth cycle of chemotherapy and was hence referred to our side for the same. Since none of the routine investigations were successful in identifying a cause for the condition, specific tests like mycoplasma pneumoniae antibodies, rheumatic factor, and antinuclear antibody tests were conducted, and the results were negative. A repeat chest radiograph revealed non-segmental patchy air space consolidation areas in the right lung’s lower zone. The high-resolution computed tomography scan of the thorax also revealed similar findings like areas of “ground glass” opacities at the base of the right lung, with patchy foci of air space consolidation, mostly in the subpleural region (Figure 1). Due to inconclusive evidence from blood and radiological investigations, a decision was made to subject the patient to bronchoscopy.
The results of the bronchoscopic examination revealed an uninflamed, normal bronchial tree with healthy mucosal covering and segmental bronchi being patent. Bronchial secretions and bronchoalveolar lavage were taken and subjected to testing. The tests conducted in terms of microbiology and cytology presented negative results, thereby ruling out any evident microbiological or malignant cause for the condition of the patient. Further tests conducted were also unable to reveal any definitive cause for the condition. Drug-induced organising pneumonia was suspected as a diagnosis of exclusion, and trastuzumab was held responsible for causing the same. Due to this, the administration of trastuzumab was halted due to concerns that the medication might contribute to illness. Steroid administration was advised to reduce the effects of drug-induced changes caused to the lungs. Initially, dexamethasone was administered orally for two weeks, and the dose was tapered over the course of a two-month treatment period and stopped. The use of steroid suggested a drastic clinical improvement in the patient. The other drug paclitaxel, which was given to the patient along with trastuzumab, was continued further. The patient showed symptomatic relief after holding back trastuzumab, hence strengthening our conviction that it is the main cause of organising pneumonia in our case.
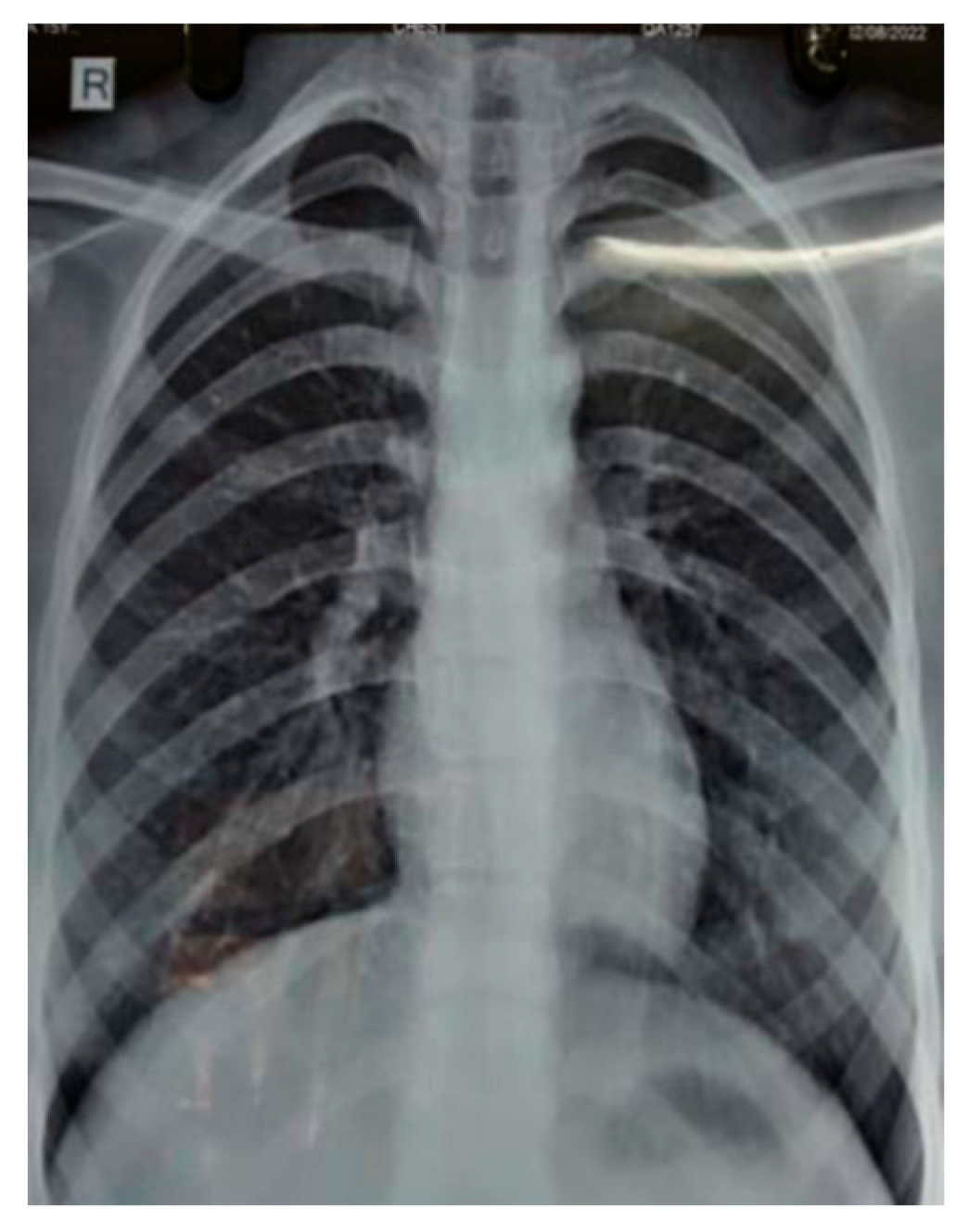
A subsequent chest X-ray suggested that the lesions had disappeared entirely (Figure 2). This further confirmed that the patient’s condition was caused by reaction of the body towards trastuzumab, and it should be noted as one of the side effects of the drug.
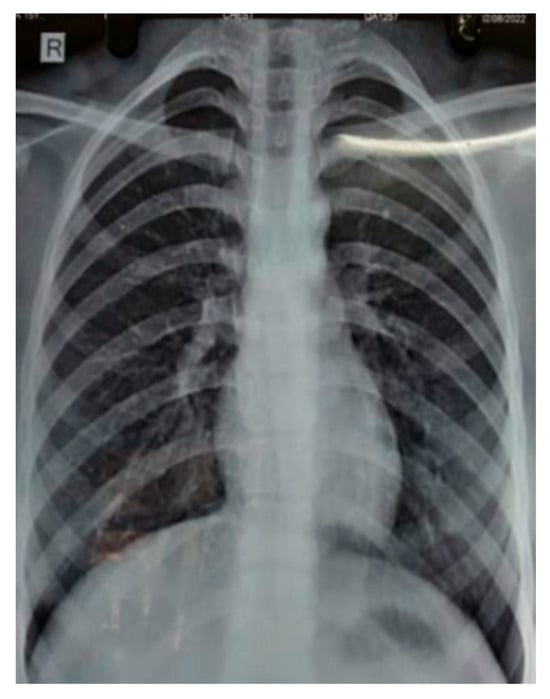
Figure 2.
Chest radiology of 58-year-old female showing resolution of right lower lobe consolidation after stopping trastuzumab therapy.
3. Discussion
Breathlessness and fever are common symptoms among patients receiving cancer treatment, and these symptoms can develop due to the progression of the malignant disease or as a result of the side effect of various chemotherapeutic or radiotherapeutic management which patients are subjected to during treatment. Our patient under study complained only of dyspnoea with no fever or increased counts, thereby placing the chances of infection under suspicion. But, radiological imaging demonstrated consolidation, which is likely due to infection. In our case, organising pneumonia was held to be secondary and differentials included infections, malignancy, trastuzumab, paclitaxel, or prior radiation. However, the absence of any demonstrable pathogen and the occurrence of pulmonary lesions outside the known extrathoracic radiation field precluded infections or radiation as a cause. However, failure to resolve symptoms and chest X-ray changes despite antimicrobial therapy, the absence of fever, and positive cultures should raise the possibility of other diagnoses. These include cancer progression, pulmonary embolism, drug-related adverse events, organising pneumonia, eosinophilic pneumonia, and pulmonary vasculitis.
Organising pneumonia or bronchiolitis obliterans organising pneumonia (BOOP) is one of the major idiopathic interstitial pneumonias (IIPs) characterised by granulation tissue within the airspaces and bronchiolar lumen that occurs as a non-specific inflammatory process. Typical radiological features include multiple alveolar opacities, usually bilateral and peripheral, often migratory, varying in size from a few centimetres to a whole lobe, with air bronchograms often present in consolidated opacities. The common symptoms with which the patient can present include: persistent dry cough, high fever, shaking and chills, shortness of breath on exertion, a loss of energy and exhaustion, weight loss, and difficulty in breathing. It can present with a variety of other symptoms as well. It has several causes, which include all types of pneumonia (including bacterial, viral, and fungal pathogens), malignancy, radiotherapy, connective tissue disease, and drug-related adverse events [3,4]. In our patient, symptoms first were presented after the fourth cycle of trastuzumab, which included breathlessness on exertion, persistent cough, and high-grade and intermittent fever. The cause could not be identified. There were no pathogens in the microbiological cultures of blood or sputum, nor did she undergo any radiotherapy. Other causes of OP were excluded. There was no clinical or serological evidence of collagen vascular disease, connective tissue disorders, and no exposure to fumes and toxins. History was taken to rule out connective tissue disorder, interstitial lung disorder, and other causes that might produce a similar picture. The pneumonitis warranted further investigations, including bronchoscopy. Bronchoalveolar lavage was negative for infectious aetiology, but the biopsy was consistent with a diagnosis of organising pneumonia. Therefore, a diagnosis of drug-induced organising pneumonia was made and the patient was put on steroids (dexamethasone) and trastuzumab was halted. She made a remarkable recovery and was sent home on hormone therapy, lapatinib, and tapering steroid dosages. The pathological findings of OP are not specific to any condition. The disease is pathologically defined by the presence of buds of granulation tissue formed by fibrin exudates, fibroblasts, myofibroblasts, and loose connective tissue in distal air spaces (alveolar spaces and bronchiolar lumen), although lung structure is preserved.
It is noted as an effect of post-drug reactions (amiodarone, nitrofurantoin, acebutolol, amphotericin, cefradin, cocaine, gold-derivate, penicillamine, sulfasalazine, sulindac, phenytoin, phenobarbital) related with injury to the lung caused by chemical substances and may be idiopathic [5,6,7]. This disease can also be observed in patients treated for neoplasm after bone marrow transplantation, bleomycin, busulfan, cyclophosphamide, methotrexate, mitomycin-C, interferon 2a and b1a treatment, and during radiotherapy [4,5,6]. Despite treatment with cyclophosphamide (known causes of BOOP), a close relationship between the administration of antibodies and the manifestation of general symptoms and pulmonary infiltrates was observed in this patient, proven by the withdrawal of trastuzumab therapy. Trastuzumab is a monoclonal antibody against human epidermal growth factor receptor 2 (HER2). Trastuzumab binds to an extracellular domain of this receptor and inhibits HER2 homodimerisation, thereby preventing HER2-mediated signalling. It is also thought to facilitate antibody-dependent cellular cytotoxicity, leading to the death of cells that express HER2 [6]. Its mechanism differs slightly from that of the newer agent pertuzumab, as the latter inhibits heterodimerisation of HER2 with HER3, a related growth factor receptor. It is an effective and well-tolerated drug. Trastuzumab-associated pneumonitis is a rare, potentially fatal side effect, presenting as hypoxemia, dyspnoea, and respiratory failure [8]. Common radiological findings are interstitial infiltrates with a ground-glass appearance and patchy foci of airspace consolidation.
Furthermore, the patient was a follow-up case of coronary artery disease, wherein echocardiography revealed an LVEF of 48% with hypokinetic RCA territory. A retrospective analysis of the side effects of trastuzumab in 25,000 patients showed that cardiotoxicity was the main side effect, but it was also connected with previous anthracyclines treatment. During the first trastuzumab infusion, there were 74 significant responses among the patients in the presented group, including bronchospasm, cardiorespiratory failure, anaphylactic shock, vasomotor oedema, and tachycardia. Nine of these patients ultimately passed away, having earlier endured respiratory failure brought on by neoplasm and related ventilatory problems. In addition, some patients had the Adult Respiratory Distress Syndrome identified, yet, in none of the situations was OP recognised.
4. Conclusions
Organising pneumonia can occur in patients with metastatic cancers. Trastuzumab is reasonably safe to use, and only about 0.3% of patients have severe reactions with features of anaphylaxis or bronchospasm, usually occurring within 2.5 h of drug administration. Trastuzumab-associated pneumonitis is a rare, potentially fatal side effect, presenting as hypoxemia, dyspnoea, and respiratory failure. Patients undergoing treatment for cancer with either chemotherapy or targeted therapy may develop drug-induced organising pneumonitis as a rare adverse reaction [9]. Clinical and radiological features closely mimic infection or progressive malignancy. Thus, a high index of suspicion is needed for diagnosis. Failure to diagnose may lead to delays in treatment and translate into deleterious outcomes. It is very important that even though by means of exclusion, the diagnosis of organising pneumonia is made so that treatment can be started as soon as possible and the disease is not left unattended. Although trastuzumab-induced pneumonitis is not very common, if induced, it can lead to deleterious outcomes. Therefore, as discussed in this case, a proper follow-up of the case is required with investigations to rule out all the infectious causes presenting with the same symptoms; the drug causing the symptoms needs to be stopped and steroid therapy needs to be started immediately to reduce the symptoms. Conditions leading to this must be identified and proper study needs to be conducted to have a thorough understanding of the disease.
Author Contributions
S.D., J.B., S.K., A.K.V. and P.P., have contributed towards the diagnosing and management of the patient. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Consent to publish has been obtained from the participant (patient) to report individual patient data.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cordier, J.F. Organizing pneumonia. Thorax 2000, 55, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Cook-Bruns, N. Retrospective analysis of the safety of Herceptin® immunotherapy in metastatic breast cancer. Oncology 2001, 61 (Suppl. 2), 58–66. [Google Scholar] [CrossRef] [PubMed]
- Nabholtz, J.M.; Slamon, D. New adjuvant strategies for breast cancer: Meeting the challenge of integrating chemotherapy and Trastuzumab (Herceptin). Semin. Oncol. 2001, 28, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Crestani, B.; Valeyre, D.; Roden, S.; Wallaert, B.; Dalphin, J.C.; Cordier, J.F.; Groupe d’Etudes et de Recherche sur les Maladies Orphelines Pulmonaires (GERM “O” P). Bronchiolitis obliterans organizing pneumonia syndrome primed by radiation therapy to the breast. Am. J. Respir. Crit. Care Med. 1998, 158, 1929–1935. [Google Scholar] [CrossRef]
- Lohr, R.H.; Boland, B.J.; Douglas, W.W.; Dockrell, D.H.; Colby, T.V.; Swensen, S.J.; Wollan, P.C.; Silverstein, M.D. Organizing pneumonia: Features and prognosis of cryptogenic, secondary, and focal variants. Arch. Intern. Med. 1997, 157, 1323–1329. [Google Scholar] [CrossRef] [PubMed]
- King, T.E.; Mortenson, R.L. Cryptogenic organizing pneumonitis: The North American experience. Chest 1992, 102, 8–13. [Google Scholar] [CrossRef]
- Arbetter, K.R.; Prakash, U.B.; Tazelaar, H.D.; Douglas, W.W. Radiation-induced pneumonitis in the “nonirradiated” lung. InMayo Clin. Proc. 1999, 74, 27–36. [Google Scholar] [CrossRef]
- Alkan, A. Interstitial pneumonitis associated with Trastuzumab emtansine. J. Oncol. Pharm. Pract. 2019, 25, 1798–1800. [Google Scholar] [CrossRef]
- Zhao, Z.; He, Z.; Huang, H.; Chen, J.; He, S.; Yilihamu, A.; Nie, Y. Drug-induced interstitial lung disease in breast cancer patients: A lesson we should learn from multi-disciplinary integration. BIO Integr. 2020, 1, 82–91. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).