Overview of Memory NK Cells in Viral Infections: Possible Role in SARS-CoV-2 Infection
Abstract
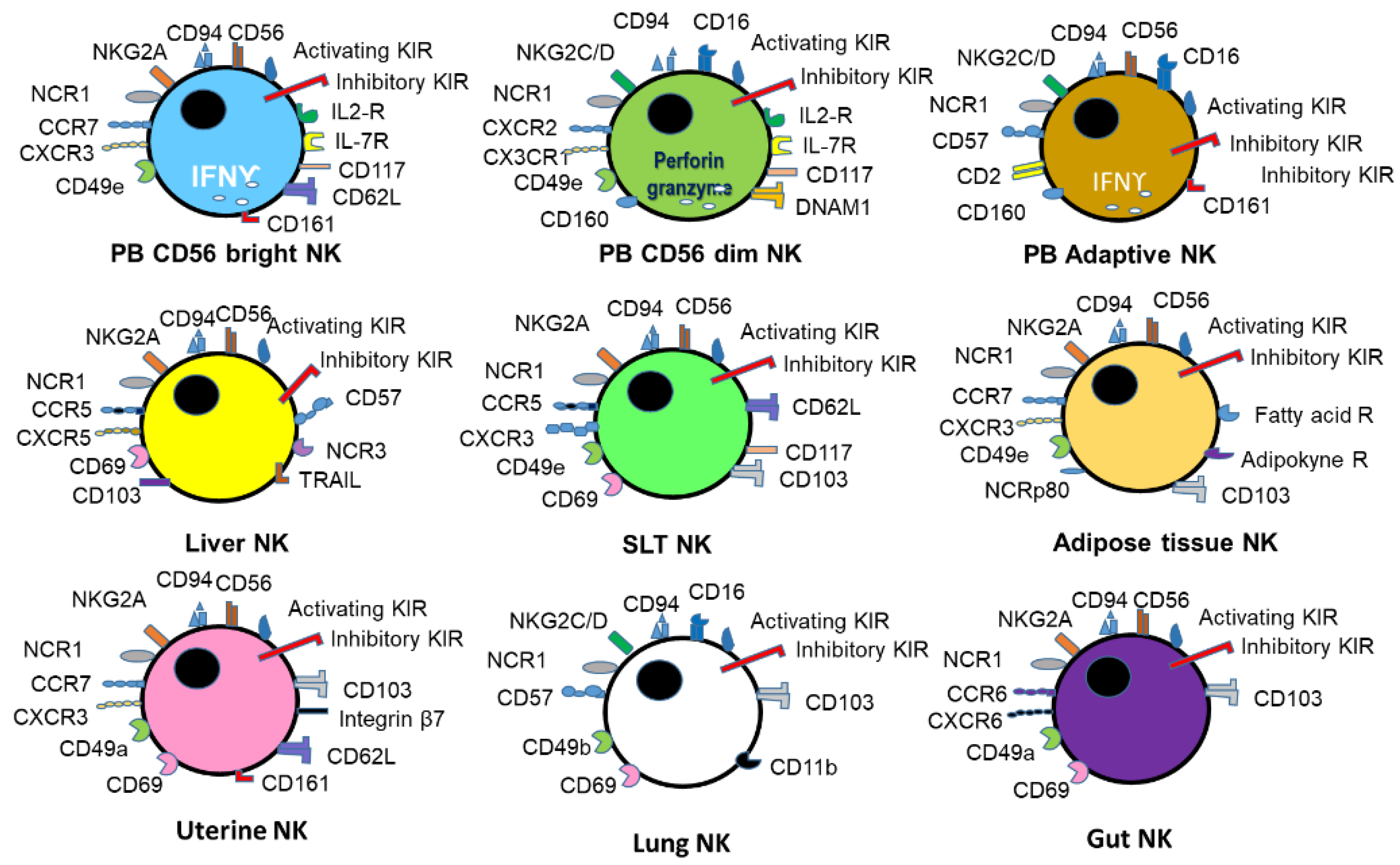
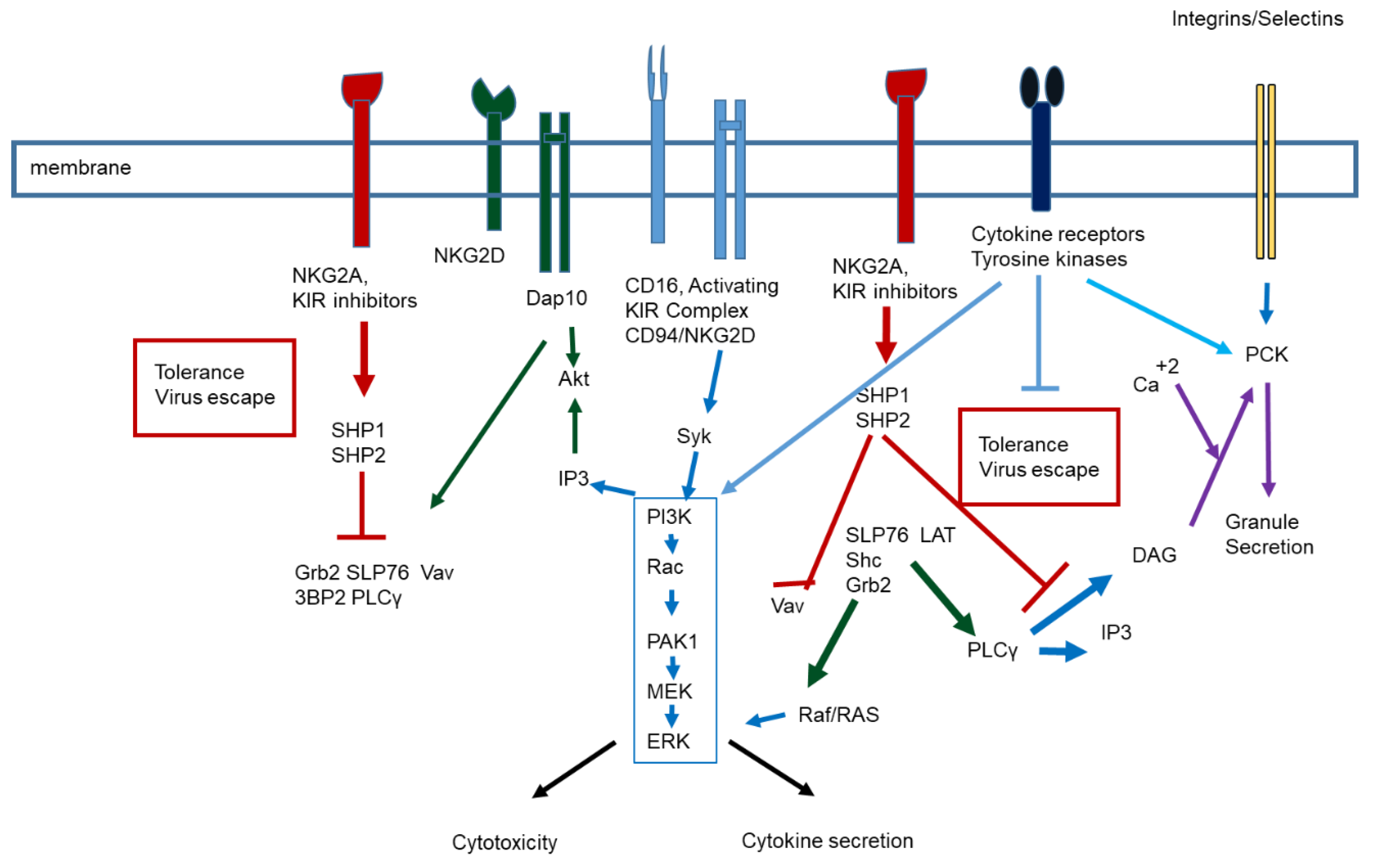
:1. NK Biology and Function
2. Viral Infection and NK Cell Response
3. SARS-CoV-2 Infection and NK Cells
3.1. Virus Infection and Immune Response
3.2. NK Cells in SARS-CoV-2 Infection
3.3. NK Cell Therapy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Caligiuri, M.A. Human natural killer cells. Blood 2008, 112, 461–469. [Google Scholar] [CrossRef]
- Freud, A.G.; Mundy-Bosse, B.L.; Yu, J.; Caligiuri, M.A. The Broad Spectrum of Human Natural Killer Cell Diversity. Immunity 2017, 47, 820–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Lu, D.; Churov, A.; Fu, R. Research Progress on NK Cell Receptors and Their Signaling Pathways. Mediat. Inflamm. 2020, 2020, 6437057. [Google Scholar] [CrossRef] [PubMed]
- Schwane, V.; Huynh-Tran, V.H.; Vollmers, S.; Yakup, V.M.; Sauter, J.; Schmidt, A.H.; Peine, S.; Altfeld, M.; Richert, L.; Körner, C. Distinct Signatures in the Receptor Repertoire Discriminate CD56bright and CD56dim Natural Killer Cells. Front. Immunol. 2020, 11, 568927. [Google Scholar] [CrossRef]
- Garmendia, J.V.; De Sanctis, J.B. A Brief Analysis of Tissue-Resident NK Cells in Pregnancy and Endometrial Diseases: The Importance of Pharmacologic Modulation. Immuno 2021, 1, 174–193. [Google Scholar] [CrossRef]
- Goodier, M.R.; Riley, E.M. Regulation of the human NK cell compartment by pathogens and vaccines. Clin. Transl. Immunol. 2021, 10, e1244. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, A.M. Memory responses by Natural Killer cells. J. Leukoc. Biol. 2018, 104, 1087–1096. [Google Scholar] [CrossRef]
- Mancini, M.; Vidal, S.M. Mechanisms of Natural Killer Cell Evasion through Viral Adaptation. Annu. Rev. Immunol. 2020, 38, 511–539. [Google Scholar] [CrossRef]
- Huntington, N.D.; Cursons, J.; Rautela, J. The cancer-natural killer cell immunity cycle. Nat. Rev. Cancer 2020, 20, 437–454. [Google Scholar] [CrossRef]
- Sojka, D.K. Uterine Natural Killer Cell Heterogeneity: Lessons from Mouse Models. Front. Immunol. 2020, 11, 290. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Tian, Z.; Peng, H. Tissue-resident NK cells and other innate lymphoid cells. Adv. Immunol. 2020, 145, 37–53. [Google Scholar]
- Hashemi, E.; Malarkannan, S. Tissue-Resident NK Cells: Development, Maturation, and Clinical Relevance. Cancers 2020, 12, 1553. [Google Scholar] [CrossRef]
- Seillet, C.; Brossay, L.; Vivier, E. Natural killers or ILC1s? That is the question. Curr. Opin. Immunol. 2021, 68, 48–53. [Google Scholar] [CrossRef]
- Karo, J.M.; Sun, J.C. Novel molecular mechanism for generating NK-cell fitness and memory. Eur. J. Immunol. 2015, 45, 1906–1915. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Salazar, C.; Sun, J.C. Coordinated Viral Control by Cytotoxic Lymphocytes Ensures Optimal Adaptive NK Cell Responses. Cell Rep. 2020, 32, 108186. [Google Scholar] [CrossRef]
- Björkström, N.K.; Strunz, B.; Ljunggren, H.G. Natural killer cells in antiviral immunity. Nat. Rev. Immunol. 2021, 1–12. [Google Scholar] [CrossRef]
- Ma, L.; Li, Q.; Cai, S.; Peng, H.; Huyan, T.; Yang, H. The role of NK cells in fighting the virus infection and sepsis. Int. J. Med. Sci. 2021, 18, 3236–3248. [Google Scholar] [CrossRef] [PubMed]
- Dębska-Zielkowska, J.; Moszkowska, G.; Zieliński, M.; Zielińska, H.; Dukat-Mazurek, A.; Trzonkowski, P.; Stefańska, K. KIR Receptors as Key Regulators of NK Cells Activity in Health and Disease. Cells 2021, 10, 1777. [Google Scholar] [CrossRef] [PubMed]
- Galitska, G.; Coscia, A.; Forni, D.; Steinbrueck, L.; De Meo, S.; Biolatti, M.; De Andrea, M.; Cagliani, R.; Leone, A.; Bertino, E.; et al. Genetic Variability of Human Cytomegalovirus Clinical Isolates Correlates with Altered Expression of Natural Killer Cell-Activating Ligands and IFN-γ. Front. Immunol. 2021, 12, 532484. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, E.G.; Dunai, C.; Judge, S.J.; Zamora, A.E.; Khuat, L.T.; Vick, L.V.; Collins, C.P.; Stoffel, K.M.; Alvarez, M.; Barao, I.; et al. Activation status dictates the function of unlicensed natural killer cells in mice and humans. Blood Adv. 2021, 5, 4219–4232. [Google Scholar] [CrossRef]
- Kared, H.; Martelli, S.; Tan, S.W.; Simoni, Y.; Chong, M.L.; Yap, S.H.; Newell, E.W.; Pender, S.L.F.; Kamarulzaman, A.; Rajasuriar, R.; et al. Adaptive NKG2C+CD57+ Natural Killer Cell and Tim-3 Expression During Viral Infections. Front. Immunol. 2018, 9, 686. [Google Scholar] [CrossRef]
- Foley, B.; Cooley, S.; Verneris, M.R.; Curtsinger, J.; Luo, X.; Waller, E.K.; Anasetti, C.; Weisdorf, D.; Miller, J.S. Human cytomegalovirus (CMV)-induced memory-like NKG2C(+) NK cells are transplantable and expand in vivo in response to recipient CMV antigen. J. Immunol. 2012, 189, 5082–5088. [Google Scholar] [CrossRef] [Green Version]
- Kobyzeva, P.A.; Streltsova, M.A.; Erokhina, S.A.; Kanevskiy, L.M.; Telford, W.G.; Sapozhnikov, A.M.; Kovalenko, E.I. CD56dim CD57- NKG2C+ NK cells retaining proliferative potential are possible precursors of CD57+ NKG2C+ memory-like NK cells. J. Leukoc. Biol. 2020, 108, 1379–1395. [Google Scholar] [CrossRef]
- Abou-Daya, K.I.; Oberbarnscheidt, M.H. Innate allorecognition in transplantation. J. Heart Lung Transplant. 2021, 40, 557–561. [Google Scholar] [CrossRef]
- Della Chiesa, M.; Sivori, S.; Carlomagno, S.; Moretta, L.; Moretta, A. Activating KIRs and NKG2C in Viral Infections: Toward NK Cell Memory? Front. Immunol. 2015, 6, 573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marquardt, N.; Kekäläinen, E.; Chen, P.; Lourda, M.; Wilson, J.N.; Scharenberg, M.; Bergman, P.; Al-Ameri, M.; Hård, J.; Mold, J.E.; et al. Unique transcriptional and protein-expression signature in human lung tissue-resident NK cells. Nat. Commun. 2019, 10, 3841. [Google Scholar] [CrossRef] [Green Version]
- Campos, C.; Pera, A.; Sanchez-Correa, B.; Alonso, C.; Lopez-Fernandez, I.; Morgado, S.; Tarazona, R.; Solana, R. Effect of age and CMV on NK cell subpopulations. Exp. Gerontol. 2014, 54, 130–137. [Google Scholar] [CrossRef]
- López-Botet, M.; Muntasell, A.; Martínez-Rodríguez, J.E.; López-Montañés, M.; Costa-García, M.; Pupuleku, A. Development of the adaptive NK cell response to human cytomegalovirus in the context of aging. Mech. Ageing Dev. 2016, 158, 23–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuo, W.; Zhao, X. Natural killer cells play an important role in virus infection control: Antiviral mechanism, subset expansion and clinical application. Clin. Immunol. 2021, 227, 108727. [Google Scholar] [CrossRef] [PubMed]
- Verweij, M.C.; Horst, D.; Griffin, B.D.; Luteijn, R.D.; Davison, A.J.; Ressing, M.E.; Wiertz, E.J. Viral inhibition of the transporter associated with antigen processing (TAP): A striking example of functional convergent evolution. PLoS Pathog. 2015, 11, e1004743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grauwet, K.; Vitale, M.; De Pelsmaeker, S.; Jacob, T.; Laval, K.; Moretta, L.; Parodi, M.; Parolini, S.; Cantoni, C.; Favoreel, H.W. Pseudorabies Virus US3 Protein Kinase Protects Infected Cells from NK Cell-Mediated Lysis via Increased Binding of the Inhibitory NK Cell Receptor CD300a. J. Virol. 2015, 90, 1522–1533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levin, L.I.; Munger, K.L.; O’Reilly, E.J.; Falk, K.I.; Ascherio, A. Primary infection with the Epstein-Barr virus and risk of multiple sclerosis. Ann. Neurol. 2010, 67, 824–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tangye, S.G. Genetic susceptibility to EBV infection: Insights from inborn errors of immunity. Hum. Genet. 2020, 139, 885–901. [Google Scholar] [CrossRef] [PubMed]
- Png, Y.T.; Yang, A.Z.Y.; Lee, M.Y.; Chua, M.J.M.; Lim, C.M. The Role of NK Cells in EBV Infection and EBV-Associated NPC. Viruses 2021, 13, 300. [Google Scholar] [CrossRef]
- Jud, A.; Kotur, M.; Berger, C.; Gysin, C.; Nadal, D.; Lünemann, A. Tonsillar CD56brightNKG2A+ NK cells restrict primary Epstein-Barr virus infection in B cells via IFN-γ. Oncotarget 2017, 8, 6130–6141. [Google Scholar] [CrossRef] [Green Version]
- Münz, C. Natural Killer Cell Responses during Human γ-Herpesvirus Infections. Vaccines 2021, 9, 655. [Google Scholar] [CrossRef]
- Shabrish, S.; Karnik, N.; Gupta, V.; Bhate, P.; Madkaikar, M. Impaired NK cell activation during acute dengue virus infection: A contributing factor to disease severity. Heliyon 2020, 6, e04320. [Google Scholar] [CrossRef]
- Zimmer, C.L.; Cornillet, M.; Solà-Riera, C.; Cheung, K.W.; Ivarsson, M.A.; Lim, M.Q.; Marquardt, N.; Leo, Y.S.; Lye, D.C.; Klingström, J.; et al. NK cells are activated and primed for skin-homing during acute dengue virus infection in humans. Nat. Commun. 2019, 10, 3897. [Google Scholar] [CrossRef] [Green Version]
- McKechnie, J.L.; Beltrán, D.; Pitti, A.; Saenz, L.; Araúz, A.B.; Vergara, R.; Harris, E.; Lanier, L.L.; Blish, C.A.; López-Vergès, S. HLA Upregulation During Dengue Virus Infection Suppresses the Natural Killer Cell Response. Front. Cell. Infect. Microbiol. 2019, 9, 268. [Google Scholar] [CrossRef] [Green Version]
- Quintino-de-Carvalho, I.L.; Gonçalves-Pereira, M.H.; Ramos, M.F.; de Aguiar Milhim, B.H.G.; Da Costa, Ú.L.; Santos, É.G.; Nogueira, M.L.; Da Costa Santiago, H. ILC1 and NK cells are sources of IFN-γ and other inflammatory cytokines associated to distinct clinical presentation in early dengue infection. J. Infect. Dis. 2021, jiab312. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, R.R. Age-related alterations in immune responses to West Nile virus infection. Clin. Exp. Immunol. 2017, 187, 26–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, Y.; Strauss-Albee, D.M.; Zhou, J.Q.; Malawista, A.; Garcia, M.N.; Murray, K.O.; Blish, C.A.; Montgomery, R.R. The natural killer cell response to West Nile virus in young and old individuals with or without a prior history of infection. PLoS ONE 2017, 12, e0172625. [Google Scholar]
- Luczo, J.M.; Ronzulli, S.L.; Tompkins, S.M. Influenza A Virus Hemagglutinin and Other Pathogen Glycoprotein Interactions with NK Cell Natural Cytot.oxicity Receptors NKp46, NKp44, and NKp30. Viruses 2021, 13, 156. [Google Scholar] [CrossRef]
- Prigge, A.D.; Ma, R.; Coates, B.M.; Singer, B.D.; Ridge, K.M. Age-Dependent Differences in T-Cell Responses to Influenza A Virus. Am. J. Respir. Cell Mol. Biol. 2020, 63, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Achdout, H.; Vitner, E.B.; Politi, B.; Melamed, S.; Yahalom-Ronen, Y.; Tamir, H.; Erez, N.; Avraham, R.; Weiss, S.; Cherry, L.; et al. Increased lethality in influenza and SARS-CoV-2 coinfection is prevented by influenza immunity but not SARS-CoV-2 immunity. Nat. Commun. 2021, 12, 5819. [Google Scholar] [CrossRef]
- Hulme, K.D.; Noye, E.C.; Short, K.R.; Labzin, L.I. Dysregulated Inflammation during Obesity: Driving Disease Severity in Influenza Virus and SARS-CoV-2 Infections. Front. Immunol. 2021, 12, 770066. [Google Scholar] [CrossRef]
- Pittet, L.F.; Messina, N.L.; Gardiner, K.; Orsini, F.; Abruzzo, V.; Bannister, S.; Bonten, M.; Campbell, J.L.; Croda, J.; Dalcolmo, M.; et al. BCG vaccination to reduce the impact of COVID-19 in healthcare workers: Protocol for a randomised controlled trial (BRACE trial). BMJ Open 2021, 11, e052101. [Google Scholar] [CrossRef]
- Zheng, J.; Wen, L.; Yen, H.L.; Liu, M.; Liu, Y.; Teng, O.; Wu, W.F.; Ni, K.; Lam, K.T.; Huang, C.; et al. Phenotypic and Functional Characteristics of a Novel Influenza Virus Hemagglutinin-Specific Memory NK Cell. J. Virol. 2021, 95, e00165-21. [Google Scholar] [CrossRef]
- Wagstaffe, H.R.; Clutterbuck, E.A.; Bockstal, V.; Stoop, J.N.; Luhn, K.; Douoguih, M.; Shukarev, G.; Snape, M.D.; Pollard, A.J.; Riley, E.M.; et al. Ebola virus glycoprotein stimulates IL-18-dependent natural killer cell responses. J. Clin. Investig. 2020, 130, 3936–3946. [Google Scholar] [CrossRef] [Green Version]
- Jarahian, M.; Marstaller, K.; Banna, N.; Ahani, R.; Etemadzadeh, M.H.; Boller, L.K.; Azadmanesh, K.; Cid-Arregui, A.; Khezri, A.; Berger, M.R.; et al. Activating Natural Killer Cell Receptors, Selectins, and Inhibitory Siglecs Recognize Ebolavirus Glycoprotein. J. Innate Immun. 2021, 1–13. [Google Scholar] [CrossRef]
- Cox, A.; Cevik, H.; Feldman, H.A.; Canaday, L.M.; Lakes, N.; Waggoner, S.N. Targeting natural killer cells to enhance vaccine responses. Trends Pharmacol. Sci. 2021, 42, 789–801. [Google Scholar] [CrossRef]
- Corado, J.; Toro, F.; Rivera, H.; Bianco, N.E.; Deibis, L.; De Sanctis, J.B. Impairment of natural killer (NK) cytotoxic activity in hepatitis C virus (HCV) infection. Clin. Exp. Immunol. 1997, 109, 451–457. [Google Scholar] [CrossRef]
- Toro, F.; Conesa, A.; Garcia, A.; Bianco, N.E.; De Sanctis, J.B. Increased peroxide production by polymorphonuclear cells of chronic hepatitis C virus-infected patients. Clin. Immunol. Immunopathol. 1998, 88, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Marino, R.; Deibis, L.; De Sanctis, J.B.; Bianco, N.E.; Toro, F. Interaction of immune complexes isolated from hepatitis C virus-infected individuals with human cell lines. Med. Microbiol. Immunol. 2005, 194, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Adenugba, A.; Hornung, M.; Weigand, K.; Peschel, G.; Junger, H.; Kupke, P.; Lang, H.; Marquardt, J.U.; Zimmermann, T.; Geissler, E.K.; et al. Ribavirin Improves NK Cell IFNγ Response During Sofosbuvir-based DAA Therapy in HCV-infected Liver Transplant Recipients. Transplantation 2021, 105, 2226–2238. [Google Scholar] [CrossRef] [PubMed]
- Velásquez, D.; Mijares, M.; Toro, F.; Blanca, I.; De Sanctis, J.B. Efect of E2 Protein of Hepatitis C virus on NK cell activation. Rev. Fac. Med. UCV 2012, 35, 40–45. [Google Scholar]
- Doyle, E.H.; Aloman, C.; El-Shamy, A.; Eng, F.; Rahman, A.; Klepper, A.L.; Haydel, B.; Florman, S.S.; Fiel, M.I.; Schiano, T.; et al. A subset of liver resident natural killer cells is expanded in hepatitis C-infected patients with better liver function. Sci. Rep. 2021, 11, 1551. [Google Scholar] [CrossRef] [PubMed]
- Flórez-Álvarez, L.; Hernandez, J.C.; Zapata, W. NK Cells in HIV-1 Infection: From Basic Science to Vaccine Strategies. Front. Immunol. 2018, 9, 2290. [Google Scholar] [CrossRef]
- Kiani, Z.; Bruneau, J.; Geraghty, D.E.; Bernard, N.F. HLA-F on Autologous HIV-Infected Cells Activates Primary NK Cells Expressing the Activating Killer Immunoglobulin-Like Receptor KIR3DS1. J. Virol. 2019, 93, e00933-19. [Google Scholar] [CrossRef] [Green Version]
- Van de Wijer, L.; van der Heijden, W.A.; Ter Horst, R.; Jaeger, M.; Trypsteen, W.; Rutsaert, S.; van Cranenbroek, B.; van Rijssen, E.; Joosten, I.; Joosten, L.; et al. The Architecture of Circulating Immune Cells Is Dysregulated in People Living With HIV on Long Term Antiretroviral Treatment and Relates With Markers of the HIV-1 Reservoir, Cytomegalovirus, and Microbial Translocation. Front. Immunol. 2021, 12, 661990. [Google Scholar] [CrossRef]
- Christensen-Quick, A.; Massanella, M.; Frick, A.; Rawlings, S.A.; Spina, C.; Vargas-Meneses, M.; Schrier, R.; Nakazawa, M.; Anderson, C.; Gianella, S. Subclinical Cytomegalovirus DNA Is Associated with CD4 T Cell Activation and Impaired CD8 T Cell CD107a Expression in People Living with HIV despite Early Antiretroviral Therapy. J. Virol. 2019, 93, e00179-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couturier, J.; Lewis, D.E. HIV Persistence in Adipose Tissue Reservoirs. Curr. HIV/AIDS Rep. 2018, 15, 60–71. [Google Scholar] [CrossRef]
- Littwitz-Salomon, E.; Moreira, D.; Frost, J.N.; Choi, C.; Liou, K.T.; Ahern, D.K.; O’Shaughnessy, S.; Wagner, B.; Biron, C.A.; Drakesmith, H.; et al. Metabolic requirements of NK cells during the acute response against retroviral infection. Nat. Commun. 2021, 12, 5376. [Google Scholar] [CrossRef]
- Lamarche, B.J.; Orazio, N.I.; Goben, B.; Meisenhelder, J.; You, Z.; Weitzman, M.D.; Hunter, T. Repair of protein-linked DNA double strand breaks: Using the adenovirus genome as a model substrate in cell-based assays. DNA Repair 2019, 74, 80–90. [Google Scholar] [CrossRef]
- Sester, M.; Ruszics, Z.; Mackley, E.; Burgert, H.G. The transmembrane domain of the adenovirus E3/19K protein acts as an endoplasmic reticulum retention signal and contributes to intracellular sequestration of major histocompatibility complex class I molecules. J. Virol. 2013, 87, 6104–6117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Sanctis, J.B.; García, A.H.; Moreno, D.; Hajduch, M. Coronavirus infection: An immunologists’ perspective. Scand. J. Immunol. 2021, 93, e13043. [Google Scholar] [CrossRef] [PubMed]
- Varchetta, S.; Mele, D.; Oliviero, B.; Mantovani, S.; Ludovisi, S.; Cerino, A.; Bruno, R.; Castelli, A.; Mosconi, M.; Vecchia, M.; et al. Unique immunological profile in patients with COVID-19. Cell. Mol. Immunol. 2021, 18, 604–612. [Google Scholar] [CrossRef]
- Becker, C.; Reinhardt, C. Unexpected role of natural killer cell-derived interferon-γ as a driver of NETosis and DVT. J. Thromb. Haemost. 2021, 17, 400–402. [Google Scholar] [CrossRef] [Green Version]
- Maucourant, C.; Filipovic, I.; Ponzetta, A.; Aleman, S.; Cornillet, M.; Hertwig, L.; Strunz, B.; Lentini, A.; Reinius, B.; Brownlie, D.; et al. Natural killer cell immunotypes related to COVID-19 disease severity. Sci. Immunol. 2020, 5, eabd6832. [Google Scholar] [CrossRef]
- Kim, H.; Byun, J.E.; Yoon, S.R.; Koohy, H.; Jung, H.; Choi, I. SARS-CoV-2 peptides bind to NKG2D and increase NK cell activity. Cell. Immunol. 2021, 371, 104454. [Google Scholar] [CrossRef] [PubMed]
- Mukund, K.; Nayak, P.; Ashokkumar, C.; Rao, S.; Almeda, J.; Betancourt-Garcia, M.M.; Sindhi, R.; Subramaniam, S. Immune Response in Severe and Non-Severe Coronavirus Disease 2019 (COVID-19) Infection: A Mechanistic Landscape. Front. Immunol. 2021, 12, 738073. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, W.C.; Lai, E.Y.; Liu, Y.T.; Wang, Y.F.; Tzeng, Y.S.; Cui, L.; Lai, Y.J.; Huang, H.C.; Huang, J.H.; Ni, H.C.; et al. NK cell receptor and ligand composition influences the clearance of SARS-CoV-2. J. Clin. Investig. 2021, 131, e146408. [Google Scholar] [CrossRef]
- Krämer, B.; Knoll, R.; Bonaguro, L.; ToVinh, M.; Raabe, J.; Astaburuaga-García, R.; Schulte-Schrepping, J.; Kaiser, K.M.; Rieke, G.J.; Bischoff, J.; et al. Early IFN-α signatures and persistent dysfunction are distinguishing features of NK cells in severe COVID-19. Immunity 2021, 54, 2650–2669.e14. [Google Scholar] [CrossRef]
- Bastard, P.; Orlova, E.; Sozaeva, L.; Lévy, R.; James, A.; Schmitt, M.M.; Ochoa, S.; Kareva, M.; Rodina, Y.; Gervais, A.; et al. Preexisting autoantibodies to type I IFNs underlie critical COVID-19 pneumonia in patients with APS-1. J. Exp. Med. 2021, 218, e20210554. [Google Scholar] [CrossRef]
- Raadsen, M.P.; Gharbharan, A.; Jordans, C.C.E.; Mykytyn, A.Z.; Lamers, M.M.; van den Doel, P.B.; Endeman, H.; van den Akker, J.P.C.; Geurtsvan Kessel, C.H.; Koopmans, M.P.G.; et al. Interferon-α2 Auto-antibodies in Convalescent Plasma Therapy for COVID-19. J. Clin. Immunol. 2021. [Google Scholar] [CrossRef]
- Brusilovsky, M.; Cordoba, M.; Rosental, B.; Hershkovitz, O.; Andrake, M.D.; Pecherskaya, A.; Einarson, M.B.; Zhou, Y.; Braiman, A.; Campbell, K.S.; et al. Genome-wide siRNA screen reveals a new cellular partner of NK cell receptor KIR2DL4: Heparan sulfate directly modulates KIR2DL4-mediated responses. J. Immunol. 2013, 191, 5256–5267. [Google Scholar] [CrossRef] [Green Version]
- Chittum, J.E.; Sankaranarayanan, N.V.; O’Hara, C.P.; Desai, U.R. On the Selectivity of Heparan Sulfate Recognition by SARS-CoV-2 Spike Glycoprotein. ACS Med. Chem. Lett. 2021, 12, 1710–1717. [Google Scholar] [CrossRef] [PubMed]
- Mayfosh, A.J.; Goodall, K.J.; Nguyen, T.; Baschuk, N.; Hulett, M.D. Heparanase is a regulator of natural killer cell activation and cytotoxicity. J. Leukoc. Biol. 2021. [Google Scholar] [CrossRef]
- De Sanctis, J.B.; Arciniegas, E.; Bianco, N.E. Lipoprotein lipase protects bovine endothelial cells from human NK cytotoxic activity. Cell. Immunol. 2004, 227, 59–69. [Google Scholar] [CrossRef]
- Wawina-Bokalanga, T.; Vanmechelen, B.; Lhermitte, V.; Martí-Carreras, J.; Vergote, V.; Koundouno, F.R.; Akoi-Boré, J.; Thom, R.; Tipton, T.; Steeds, K.; et al. Human Diversity of Killer Cell Immunoglobulin-Like Receptors and Human Leukocyte Antigen Class I Alleles and Ebola Virus Disease Outcomes. Emerg. Infect. Dis. 2021, 27, 76–84. [Google Scholar] [CrossRef]
- Rangchaikul, P.; Venketaraman, V. SARS-CoV-2 and the Immune Response in Pregnancy with Delta Variant Considerations. Infect. Dis. Rep. 2021, 13, 993–1008. [Google Scholar] [CrossRef]
- Niessl, J.; Sekine, T.; Buggert, M. T cell immunity to SARS-CoV-2. Semin. Immunol. 2021, 55, 101505. [Google Scholar] [CrossRef]
- Geng, X.; Li, M.; Cui, B.; Lu, C.; Liu, X.; Zhang, P.; Liu, B.; Ma, C.; Shen, Y.; Lu, Z. CD4+CD25+Foxp3+ regulatory T cells suppress NKG2D-mediated NK cell cytotoxicity in peripheral blood. Medicine 2019, 98, e15722. [Google Scholar] [CrossRef]
- Rajaram, S.; Canaday, L.M.; Ochayon, D.E.; Rangel, K.M.; Ali, A.; Gyurova, I.E.; Krishnamurthy, D.; Fletcher, J.S.; Reighard, S.D.; Cox, A.; et al. The Promise and Peril of Natural Killer Cell Therapies in Pulmonary Infection. Immunity 2020, 52, 887–889. [Google Scholar] [CrossRef] [PubMed]
- Jassem, J.; Marek-Trzonkowska, N.M.; Smiatacz, T.; Arcimowicz, Ł.; Papak, I.; Jassem, E.; Zaucha, J.M. Successful Treatment of Persistent SARS-CoV-2 Infection in a B-Cell Depleted Patient with Activated Cytotoxic T and NK Cells: A Case Report. Int. J. Mol. Sci. 2021, 22, 10934. [Google Scholar] [CrossRef]
- García-García, I.; Guerra-García, P.; Ferreras, C.; Borobia, A.M.; Carcas, A.J.; Queiruga-Parada, J.; Vicario, J.L.; Mirones, I.; Solano, C.; Eguizabal, C.; et al. A phase I/II dose-escalation multi-center study to evaluate the safety of infusion of natural killer cells or memory T cells as adoptive therapy in coronavirus pneumonia and/or lymphopenia: RELEASE study protocol. Trials 2021, 22, 674. [Google Scholar] [CrossRef] [PubMed]
- Herrera, L.; Martin-Inaraja, M.; Santos, S.; Inglés-Ferrándiz, M.; Azkarate, A.; Perez-Vaquero, M.A.; Vesga, M.A.; Vicario, J.L.; Soria, B.; Solano, C.; et al. Identifying SARS-CoV-2 “memory” NK cells from COVID-19 convalescent donors for adoptive cell therapy. Immunology 2021. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.T.; Badeti, S.; Chen, C.H.; Kim, J.; Choudhary, A.; Honnen, B.; Reichman, C.; Calianese, D.; Pinter, A.; Jiang, Q.; et al. CAR-NK Cells Effectively Target SARS-CoV-2-Spike-Expressing Cell Lines In Vitro. Front. Immunol. 2021, 12, 652223. [Google Scholar] [CrossRef]
- Wang, C.; Cui, A.; Bukenya, M.; Aung, A.; Pradhan, D.; Whittaker, C.A.; Agarwal, Y.; Thomas, A.; Liang, S.; Amlashi, P.; et al. Reprogramming NK cells and macrophages via combined antibody and cytokine therapy primes tumors for elimination by checkpoint blockade. Cell Rep. 2021, 37, 110021. [Google Scholar] [CrossRef]
- Mazzitelli, I.; Bleichmar, L.; Ludueña, M.G.; Pisarevsky, A.; Labato, M.; Chiaradia, V.; Finocchieto, P.; Paulin, F.; Hormanstorfer, M.; Baretto, M.C.; et al. Immunoglobulin G Immune Complexes May Contribute to Neutrophil Activation in the Course of Severe Coronavirus Disease 2019. J. Infect. Dis. 2021, 224, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Rostad, C.A.; Anderson, L.J.; Sun, H.Y.; Lapp, S.A.; Stephens, K.; Hussaini, L.; Gibson, T.; Rouphael, N.; Anderson, E.J. The development and kinetics of functional antibody-dependent cell-mediated cytotoxicity (ADCC) to SARS-CoV-2 spike protein. Virology 2021, 559, 1–9. [Google Scholar] [CrossRef]
- Chakraborty, S.; Gonzalez, J.; Edwards, K.; Mallajosyula, V.; Buzzanco, A.S.; Sherwood, R.; Buffone, C.; Kathale, N.; Providenza, S.; Xie, M.M.; et al. Proinflammatory IgG Fc structures in patients with severe COVID-19. Nat. Immunol. 2021, 22, 67–73. [Google Scholar] [CrossRef]
- Goodier, M.R.; Lusa, C.; Sherratt, S.; Rodriguez-Galan, A.; Behrens, R.; Riley, E.M. Sustained Immune Complex-Mediated Reduction in CD16 Expression after Vaccination Regulates NK Cell Function. Front. Immunol. 2016, 7, 384. [Google Scholar] [CrossRef] [Green Version]
- Chadchan, S.B.; Popli, P.; Maurya, V.K.; Kommagani, R. The SARS-CoV-2 receptor, angiotensin-converting enzyme 2, is required for human endometrial stromal cell decidualization. Biol. Reprod. 2021, 104, 336–343. [Google Scholar] [CrossRef]
- Shmeleva, E.V.; Colucci, F. Maternal natural killer cells at the intersection between reproduction and mucosal immunity. Mucosal Immunol. 2021, 14, 991–1005. [Google Scholar] [CrossRef]
- Jorgensen, S.C.; Burry, L.; Tabbara, N. The role of maternal COVID-19 vaccination in providing immunological protection to the newborn. Pharmacotherapy 2021. [Google Scholar] [CrossRef]
- Li, M.; Guo, W.; Dong, Y.; Wang, X.; Dai, D.; Liu, X.; Wu, Y.; Li, M.; Zhang, W.; Zhou, H.; et al. Elevated Exhaustion Levels of NK and CD8+ T Cells as Indicators for Progression and Prognosis of COVID-19 Disease. Front. Immunol. 2020, 11, 580237. [Google Scholar] [CrossRef]
- Saitoh, S.; Van Wijk, K.; Nakajima, O. Crosstalk between Metabolic Disorders and Immune Cells. Int. J. Mol. Sci. 2021, 22, 10017. [Google Scholar] [CrossRef]
- Hsu, A.T.; Gottschalk, T.A.; Tsantikos, E.; Hibbs, M.L. The Role of Innate Lymphoid Cells in Chronic Respiratory Diseases. Front. Immunol. 2021, 12, 733324. [Google Scholar] [CrossRef] [PubMed]
- Delaney, J.A.C.; Olson, N.C.; Sitlani, C.M.; Fohner, A.E.; Huber, S.A.; Landay, A.L.; Heckbert, S.R.; Tracy, R.P.; Psaty, B.M.; Feinstein, M.; et al. Natural killer cells, gamma delta T cells and classical monocytes are associated with systolic blood pressure in the multi-ethnic study of atherosclerosis (MESA). BMC Cardiovasc. Disord. 2021, 21, 45. [Google Scholar] [CrossRef]
- Gardner, G.; Fraker, C.A. Natural Killer Cells as Key Mediators in Type I Diabetes Immunopathology. Front Immunol. 2021, 12, 722979. [Google Scholar] [CrossRef] [PubMed]
- Habib, H.M.; Ibrahim, S.; Zaim, A.; Ibrahim, W.H. The role of iron in the pathogenesis of COVID-19 and possible treatment with lactoferrin and other iron chelators. Biomed. Pharmacother. 2021, 136, 111228. [Google Scholar] [CrossRef] [PubMed]
- Muhoberac, B.B. What Can Cellular Redox, Iron, and Reactive Oxygen Species Suggest About the Mechanisms and Potential Therapy of COVID-19? Front. Cell. Infect. Microbiol. 2020, 10, 569709. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Jiang, J.; Fu, N.; Chen, L. Targetting ferroptosis for blood cell-related diseases. J. Drug Target. 2021, 1–15. [Google Scholar] [CrossRef]
- Lange, C.; Csernok, E.; Moosig, F.; Holle, J.U. Immune stimulatory effects of neutrophil extracellular traps in granulomatosis with polyangiitis. Clin. Exp. Rheumatol. 2017, 35 (Suppl. 103), 33–39. [Google Scholar]
- Melgaço, J.G.; Azamor, T.; Silva, A.M.V.; Linhares, J.H.R.; Dos Santos, T.P.; Mendes, Y.S.; de Lima, S.M.B.; Fernandes, C.B.; da Silva, J.; de So, A.F.; et al. Two-Step In Vitro Model to Evaluate the Cellular Immune Response to SARS-CoV-2. Cells 2021, 10, 2206. [Google Scholar] [CrossRef]
- Lau, C.M.; Wiedemann, G.M.; Sun, J.C. Epigenetic regulation of natural killer cell memory. Immunol. Rev. 2021. [Google Scholar] [CrossRef] [PubMed]
| Species | NK Cell Receptor | Natural Cellular Ligand | Effect on NK Function |
|---|---|---|---|
| Mouse | NCR46 | Vimentin, viral antigen | Activating |
| GAG | |||
| NKG2D | RAE 1a, b, d, g | ||
| H60a-c, MULT1 | |||
| DNAM1 | CD112, CD155 | ||
| CD94/NKG2C | Qa-1 | ||
| CD94/NKG2E | Qa-1 | ||
| CD16 | IgG | ||
| LY49D | H2Dd | ||
| LY49H | CMV glycoprotein | ||
| Ly49P | CMV glycoprotein | ||
| CD94/NKG2A | Qa-1 | Inhibiting | |
| KLRG1 | Cadherins (E, N, and R) | ||
| LY49A | H2Dd, H2Dk | ||
| LY49I | H2Dk | ||
| NK1.1 | Lectin | ||
| CD244 | CD48 | Activating/Inhibiting | |
| Human | CD94/NKG2C CD94/NKG2D | DAP-12/HLA | Activating |
| MICA A/B, ULPB1-6 | |||
| NCR30 | B7-H6, BAT 3, GAG | ||
| NCR44 | Heparan sulfate, heparin. GAG | ||
| NCR46 | Vimentin, Viral antigens, GAG | ||
| IgG | |||
| CD16 | HLA-C2 | ||
| KIR2DS1 | |||
| KIR2DS2 | HLA-C | ||
| KIR2DS4 | HLA-C | ||
| KIR2DS5 | HLA-C | ||
| KIR3DS1 | HLA-B, HLA-F | ||
| KIR2DL4 | HLA-G | ||
| DNAM1 | CD112, CD155 | ||
| NTBA | NTB-A, viral antigens | ||
| CD94/NKG2A | HLA-E | Inhibiting | |
| KIR3DL1/2 | HLA-C | ||
| KIR2DL2/3 | HLA-B/HLA-C | ||
| KIR2DL1 | HLA-C | ||
| KLRG1 | Cadherins (E, N, and R) | ||
| ILT2 | HLA-E | ||
| CD244 | CD48 | Activating/Inhibiting |
| Species | Virus | Virus Viral Ligand or Virus-Cellular Ligands | Nk Cell Receptor |
|---|---|---|---|
| Mouse | MCMV | m157, m154 | Ly49H/DAP12 |
| (C57BL/6), Ly49I (129) | |||
| H-2Dk with m04 | Ly49P/DAP12 | ||
| Virus-induced cell stress ligands: Rae1, H60a-c, Mult1b | NKG2D | ||
| m154 reduces CD48c | CD244 | ||
| Ectromelia v | Qa-1b | CD94/NKG2E | |
| Influenza A v | HA | NKp46 | |
| Human | HCMV | pp65, HLA-E. Cell stress ligands: MICA/B, ULBP1-4b, | CD94/NKG2C NKG2D |
| RL-11, UL118 LILRB1. UL40, UL18 | NKp30, FcΥR, | ||
| HSV | gE, gD, gD FcγR | KIR2DS4, KIR2DS2, | |
| Pseudorabies v | gD, CD300a | KIR3DS1 | |
| EBV | vIL10, HLA-C, HA | NKp30, NKp40, NKp46, | |
| KSV | v/MIP-II, K5, | NKG2D | |
| Vaccinia v | HA | CD112 (DNAM-1 L). | |
| Dengue v | Envelope-protein | KIR2DS1, NKG2D | |
| West Nile | Envelope-protein, Hemagglutinin (HA) | CXCR3/CCR3 receptors, | |
| Influenza A v | HA-neuraminidase | NKG2D | |
| Parainfluenza | HA-neuraminidase | NKp30, NKp46 | |
| Sendai v | HA, HA-neuraminidase | NKp44 | |
| Newcastle v | HA, HA-neuraminidase | NKp44, | |
| Ebola v | Viral Glycoprotein | NKp46 | |
| HCV | E-2 protein, Scavenger receptor, NS3, CD81 | NKp46 | |
| HIV | HLA-B | NKp44, NKp46 | |
| Vpu reduces | NKp44, NKp46 | ||
| NTB-A | NKp30 | ||
| Adenovirus 5 | E3/19K | NKp30, NKp46, | |
| Papilloma V | E6, E7 | CD94/NKG2C | |
| KIR2DL1/KIR2DL3 | |||
| KIR3DS1, NKp44, NKp46 | |||
| NTB-A | |||
| NKG2D | |||
| KIR2DL1, KIR2DL2, | |||
| KIR2DL3 |
| Virus Infection-Induced Memory | NK Cell Receptor | NK Peripheral | Tissue-Resident NK Cells |
|---|---|---|---|
| CMV | CD94/NKG2C+ CD57+, KIR2DS4, KIR2DS2, KIR3DS1. | Increased peripheral NK cells in elderly individuals. Induction of CD57+ from CD56dim CD57-cells | Impairment of tissue to peripheral ILC cells |
| Dengue virus | CD94/NKG2C+. Inhibition of memory through KIR3DL1 by NS1 viral protein. | It increased peripheral CD56 bright cells. | Skin homing CLA+ NK cell phenotype |
| Ebola virus | CD94/NKG2C+ CD57+ | Increase in CD56 neg CD16pos supopulations | Active liver NK cells |
| HIV | CD94/NKG2C+ CD57+ | Increased frequencies of CD16pos CD56 ng NK cells | Lymph node, liver, placenta activated by infected cells |
| Hepatitis C | CD57+ KLRG1+ | Increased NCR46, CD56 bright | Active liver NK cells |
| Influenza virus | CD16+CD49a+CXCR3+ | Reduced CD56 bright | Increase of lung NK cells |
| SARS-CoV-2 | CD56di, NKG2C, Ksp37+ | Increase of CD56dim CD57+ cells | Active lung NK cells, Decidual, liver |
| Memory | NK Cell Receptor | Notes |
|---|---|---|
| Induced by virus | CD94/NKG2C+ CD57+ | HCMV induced memory. Present in young individuals, less probable on elders. Virus-induced mature NK cells undergo homeostatic cell division. They enhanced cytotoxic response and ADCC. |
| Infection | KIR2DS4, KIR2DS2, | |
| Human | KIR3DS1 | |
| Mouse | Ly49H+/DAP12 | NK cells that quickly respond to virus challenge. Other virus-binding receptors may be involved. |
| DNAM-1 | ||
| CXCR6+ | ||
| Cytokine-induced memory | IL-12R, IL-15R, IL-18R | Stimulation with IL-12, IL-15, IL-18 cytokines induces a pool of long-lived NK cells with enhanced cytokine reactivity and can kill virus cells. Increase ADCC. |
| Mouse and Human | ||
| Vaccine-induced | NKG2D + CXCR6+ | Influenza, BcG, Ebola, SARS-CoV-2 |
| Memory | ||
| Mouse and human |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Sanctis, J.B.; Garmendia, J.V.; Hajdúch, M. Overview of Memory NK Cells in Viral Infections: Possible Role in SARS-CoV-2 Infection. Immuno 2022, 2, 52-67. https://doi.org/10.3390/immuno2010005
De Sanctis JB, Garmendia JV, Hajdúch M. Overview of Memory NK Cells in Viral Infections: Possible Role in SARS-CoV-2 Infection. Immuno. 2022; 2(1):52-67. https://doi.org/10.3390/immuno2010005
Chicago/Turabian StyleDe Sanctis, Juan Bautista, Jenny Valentina Garmendia, and Marián Hajdúch. 2022. "Overview of Memory NK Cells in Viral Infections: Possible Role in SARS-CoV-2 Infection" Immuno 2, no. 1: 52-67. https://doi.org/10.3390/immuno2010005
APA StyleDe Sanctis, J. B., Garmendia, J. V., & Hajdúch, M. (2022). Overview of Memory NK Cells in Viral Infections: Possible Role in SARS-CoV-2 Infection. Immuno, 2(1), 52-67. https://doi.org/10.3390/immuno2010005






