Poliosis Is Associated with Response to Checkpoint-Inhibitor Therapy: A Case Report of Two Patients with Multifocal Metastatic Melanoma
Abstract
:1. Introduction
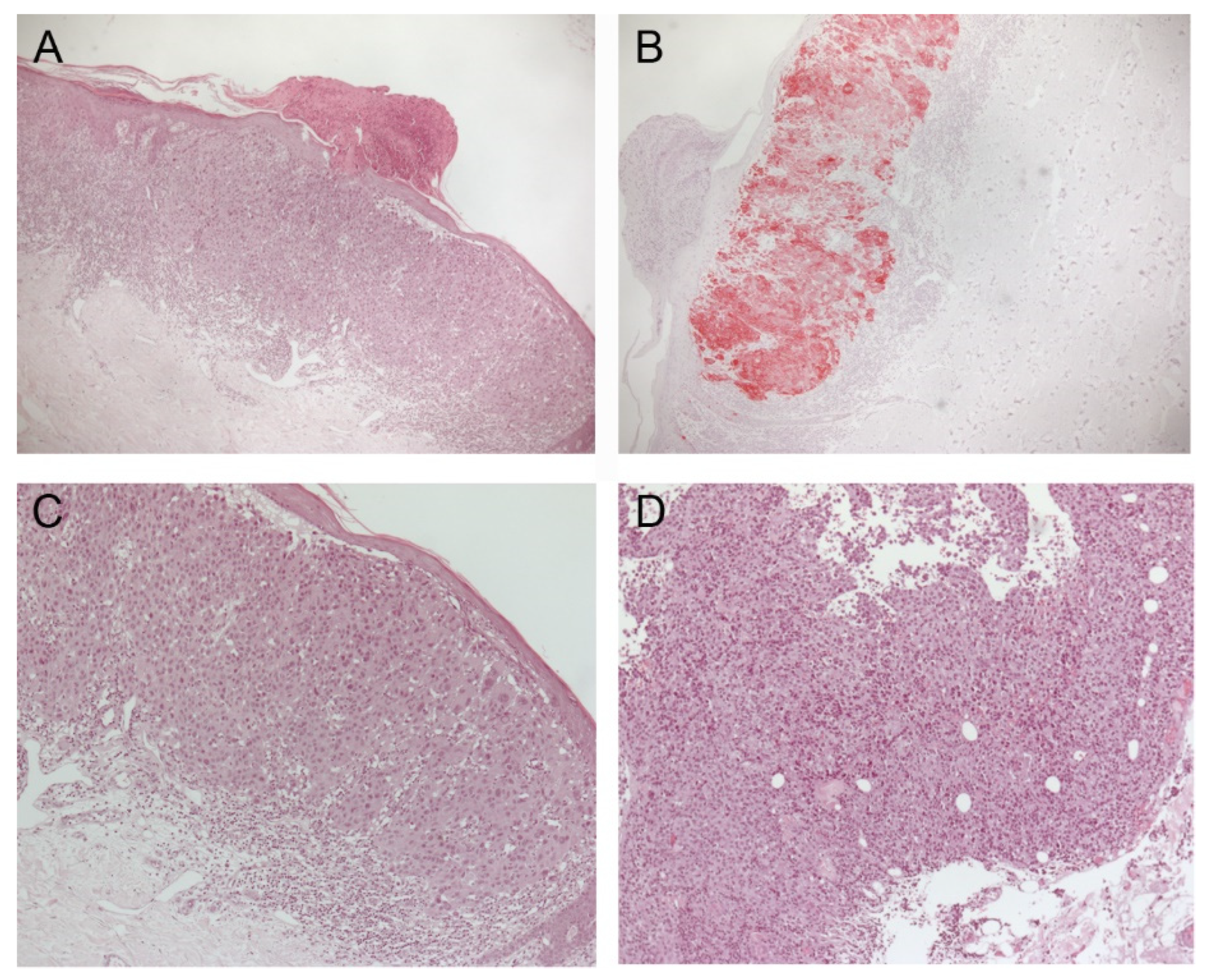
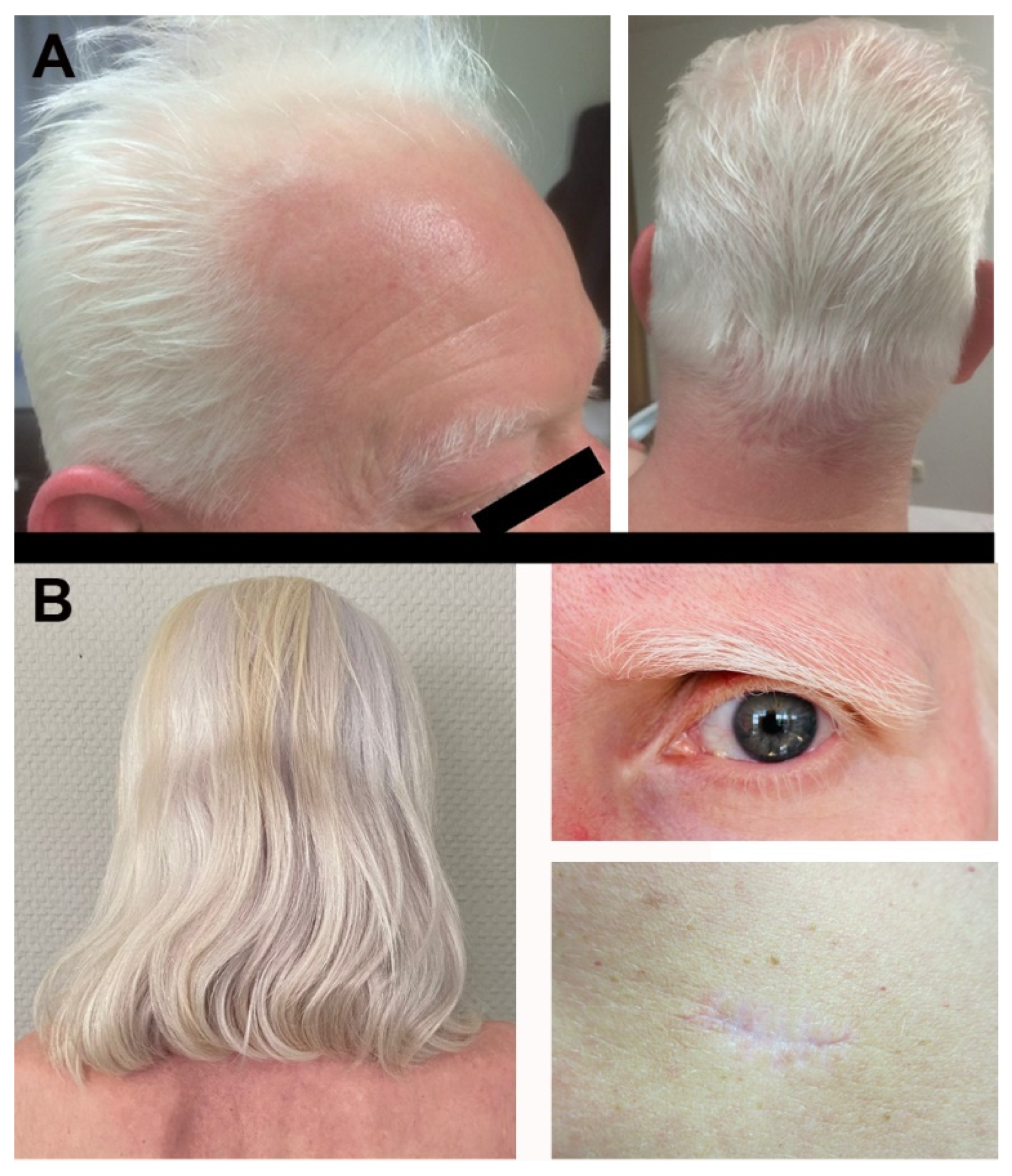
2. Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hodi, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1480–1492. [Google Scholar] [CrossRef]
- Matull, J.; Livingstone, E.; Wetter, A.; Zimmer, L.; Zaremba, A.; Lahner, H.; Schadendorf, D.; Ugurel, S. Durable Complete Response in a Melanoma Patient with Unknown Primary, Associated with Sequential and Severe Multi-Organ Toxicity After a Single Dose of CTLA-4 Plus PD-1 Blockade: A Case Report. Front. Oncol. 2020, 10, 592609. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, D.J.; Carlino, M.S. Immune Checkpoint Inhibitor Toxicity. Curr. Oncol. Rep. 2018, 20, 72. [Google Scholar] [CrossRef]
- Gault, A.; Anderson, A.E.; Plummer, R.; Stewart, C.; Pratt, A.G.; Rajan, N. Cutaneous immune-related adverse events in patients with melanoma treated with checkpoint inhibitors. Br. J. Dermatol. 2021, 185, 263–271. [Google Scholar] [CrossRef]
- Mandalà, M.; Larkin, J.M.G.; Ascierto, P.A.; Del Vecchio, M.; Gogas, H.; Cowey, C.L.; Arance Fernandez, A.N.A.M.; Dalle, S.; Schenker, M.; Grob, J.J.; et al. An analysis of nivolumab-mediated adverse events and association with clinical efficacy in resected stage III or IV melanoma (CheckMate 238). J. Clin. Oncol. 2019, 37, 9584. [Google Scholar] [CrossRef]
- Weber, J.S.; Hodi, F.S.; Wolchok, J.D.; Topalian, S.L.; Schadendorf, D.; Larkin, J.; Sznol, M.; Long, G.V.; Li, H.; Waxman, I.M.; et al. Safety Profile of Nivolumab Monotherapy: A Pooled Analysis of Patients with Advanced Melanoma. J. Clin. Oncol. 2017, 35, 785–792. [Google Scholar] [CrossRef]
- Robert, C.; Hwu, W.J.; Hamid, O.; Ribas, A.; Weber, J.S.; Daud, A.I.; Hodi, F.S.; Wolchok, J.D.; Mitchell, T.C.; Hersey, P.; et al. Long-term safety of pembrolizumab monotherapy and relationship with clinical outcome: A landmark analysis in patients with advanced melanoma. Eur. J. Cancer 2021, 144, 182–191. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Kicinski, M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.; Dalle, S.; Haydon, A.; Khattak, A.; Carlino, M.S.; et al. Association Between Immune-Related Adverse Events and Recurrence-Free Survival among Patients with Stage III Melanoma Randomized to Receive Pembrolizumab or Placebo: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2020, 6, 519–527. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, R.J.; Weber, J.S. Immune-related toxicities of checkpoint inhibitors: Mechanisms and mitigation strategies. Nat. Rev. Drug Discov. 2021. [Google Scholar] [CrossRef]
- Hofmann, L.; Forschner, A.; Loquai, C.; Goldinger, S.M.; Zimmer, L.; Ugurel, S.; Schmidgen, M.I.; Gutzmer, R.; Utikal, J.S.; Goppner, D.; et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur. J. Cancer 2016, 60, 190–209. [Google Scholar] [CrossRef] [PubMed]
- Burzi, L.; Alessandrini, A.M.; Quaglino, P.; Piraccini, B.M.; Dika, E.; Ribero, S. Cutaneous Events Associated with Immunotherapy of Melanoma: A Review. J. Clin. Med. 2021, 10, 3047. [Google Scholar] [CrossRef] [PubMed]
- Teulings, H.E.; Limpens, J.; Jansen, S.N.; Zwinderman, A.H.; Reitsma, J.B.; Spuls, P.I.; Luiten, R.M. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: A systematic review and meta-analysis. J. Clin. Oncol. 2015, 33, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Alsuhaibani, A.H.; Alhumayed, M. Primary orbital melanoma with poliosis and a palpable mass. Arch. Ophthalmol. 2011, 129, 1382–1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ediriwickrema, L.S.; Liu, C.Y.; Kikkawa, D.O.; Korn, B.S. Development of Poliosis Following Checkpoint Inhibitor Treatment for Cutaneous Melanoma. Ophthalmic Plast Reconstr. Surg. 2019, 35, e121–e122. [Google Scholar] [CrossRef]
- Failla, C.M.; Carbone, M.L.; Fortes, C.; Pagnanelli, G.; D’Atri, S. Melanoma and Vitiligo: In Good Company. Int. J. Mol. Sci. 2019, 20, 5731. [Google Scholar] [CrossRef] [Green Version]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef] [Green Version]
- Hosoya, K.; Fujimoto, D.; Morimoto, T.; Kumagai, T.; Tamiya, A.; Taniguchi, Y.; Yokoyama, T.; Ishida, T.; Hirano, K.; Matsumoto, H.; et al. Association Between Early Immune-related Adverse Events and Clinical Outcomes in Patients with Non-Small Cell Lung Cancer Treated with Immune Checkpoint Inhibitors. Clin. Lung Cancer 2020, 21, e315–e328. [Google Scholar] [CrossRef]
- Wolner, Z.J.; Marghoob, A.A.; Pulitzer, M.P.; Postow, M.A.; Marchetti, M.A. A case report of disappearing pigmented skin lesions associated with pembrolizumab treatment for metastatic melanoma. Br. J. Dermatol. 2018, 178, 265–269. [Google Scholar] [CrossRef]
- Schallreuter, K.U.; Levenig, C.; Berger, J. Vitiligo and cutaneous melanoma. A case study. Dermatologica 1991, 183, 239–245. [Google Scholar] [CrossRef]
- Blessing, K.; McLaren, K.M. Histological regression in primary cutaneous melanoma: Recognition, prevalence and significance. Histopathology 1992, 20, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Aung, P.P.; Nagarajan, P.; Prieto, V.G. Regression in primary cutaneous melanoma: Etiopathogenesis and clinical significance. Lab. Investig. 2017, 97, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Del Fiore, P.; Rastrelli, M.; Dall’Olmo, L.; Cavallin, F.; Cappellesso, R.; Vecchiato, A.; Buja, A.; Spina, R.; Parisi, A.; Mazzarotto, R.; et al. Melanoma of Unknown Primary: Evaluation of the Characteristics, Treatment Strategies, Prognostic Factors in a Monocentric Retrospective Study. Front. Oncol. 2021, 11, 627527. [Google Scholar] [CrossRef] [PubMed]
- Freeman-Keller, M.; Kim, Y.; Cronin, H.; Richards, A.; Gibney, G.; Weber, J.S. Nivolumab in Resected and Unresectable Metastatic Melanoma: Characteristics of Immune-Related Adverse Events and Association with Outcomes. Clin. Cancer Res. 2016, 22, 886–894. [Google Scholar] [CrossRef] [Green Version]
- Quach, H.T.; Dewan, A.K.; Davis, E.J.; Ancell, K.K.; Fan, R.; Ye, F.; Johnson, D.B. Association of Anti-Programmed Cell Death 1 Cutaneous Toxic Effects with Outcomes in Patients with Advanced Melanoma. JAMA Oncol. 2019, 5, 906–908. [Google Scholar] [CrossRef]
- Nakamura, Y.; Tanaka, R.; Asami, Y.; Teramoto, Y.; Imamura, T.; Sato, S.; Maruyama, H.; Fujisawa, Y.; Matsuya, T.; Fujimoto, M.; et al. Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: A multi-institutional retrospective study. J. Dermatol. 2017, 44, 117–122. [Google Scholar] [CrossRef]
- Hua, C.; Boussemart, L.; Mateus, C.; Routier, E.; Boutros, C.; Cazenave, H.; Viollet, R.; Thomas, M.; Roy, S.; Benannoune, N.; et al. Association of Vitiligo with Tumor Response in Patients with Metastatic Melanoma Treated with Pembrolizumab. JAMA Dermatol. 2016, 152, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Amini Adle, M.; Chastagner, M.; Mansard, S.; Dalle, S. Image Gallery: Unilateral eyebrow depigmentation. Br. J. Dermatol. 2019, 180, e107. [Google Scholar] [CrossRef]
- Thomas, S.; Laino, A.; Sturm, R.; Nufer, K.; Lambie, D.; Shepherd, B.; Atkinson, V.; Adams, L.; Soyer, H.P.; Schaider, H. Focal regression of a primary melanoma, fading lentigines and poliosis in metastatic melanoma treated with anti-PD-1. J. Eur. Acad. Dermatol. Venereol. 2018, 32, e176–e177. [Google Scholar] [CrossRef]
- Zarbo, A.; Belum, V.R.; Sibaud, V.; Oudard, S.; Postow, M.A.; Hsieh, J.J.; Motzer, R.J.; Busam, K.J.; Lacouture, M.E. Immune-related alopecia (areata and universalis) in cancer patients receiving immune checkpoint inhibitors. Br. J. Dermatol. 2017, 176, 1649–1652. [Google Scholar] [CrossRef]
- Gambichler, T.; Seifert, C.; Lehmann, M.; Lukas, C.; Scheel, C.; Susok, L. Concurrent Vogt-Koyanagi-Harada disease and impressive response to immune checkpoint blockade in metastatic melanoma. Immunotherapy 2020, 12, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Fusumae, T.; Kamiya, K.; Maekawa, T.; Komine, M.; Murata, S.; Inoda, S.; Takahashi, R.; Kawashima, H.; Ohtsuki, M. Vogt-Koyanagi-Harada disease-like uveitis induced by vemurafenib for metastatic cutaneous malignant melanoma. J. Dermatol. 2018, 45, e159–e160. [Google Scholar] [CrossRef] [PubMed]




| Author | Primary Melanoma | Age | Tumor Stage | ICI Agent | First Signs of Poliosis | Treatment Response | Other irAE | Ref. |
|---|---|---|---|---|---|---|---|---|
| Korn et al. | right eyelid | 52 years | IIIC | Ipilimumab + Nivolumab | 2 months upon initiation | complete remission | none | [15] |
| Gault et al. | unknown | unknown | IV | Ipilimumab | 5 years | complete remission | none | [5] |
| Wolner et al. | right lower back | 60 years | IV | Pembrolizumab | 4 months upon treatment initiation | partial response | none | [19] |
| Zarbo et al. | unknown | 65 years | IV | Ipilimumab + Nivolumab | 3 months upon initiation | partial response | alopecia, hepatitis, hypothyroidism | [30] |
| Thomas et al. | right shoulder | unknown | IV | Pembrolizumab | 5 months upon initiation | complete response | non-segmental vitiligo | [29] |
| Haist et al. | unknown and upper back | 48 years and 53 years | IV | Ipilimumab + Nivolumab | After 2 and 3 cycles of cICB | complete response | hypothyroidism, vitiligo, hepatitis and colitis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haist, M.; Stege, H.; Maikranz, V.; Halley Blanco, M.; Grabbe, S.; Loquai, C. Poliosis Is Associated with Response to Checkpoint-Inhibitor Therapy: A Case Report of Two Patients with Multifocal Metastatic Melanoma. Immuno 2022, 2, 307-316. https://doi.org/10.3390/immuno2020020
Haist M, Stege H, Maikranz V, Halley Blanco M, Grabbe S, Loquai C. Poliosis Is Associated with Response to Checkpoint-Inhibitor Therapy: A Case Report of Two Patients with Multifocal Metastatic Melanoma. Immuno. 2022; 2(2):307-316. https://doi.org/10.3390/immuno2020020
Chicago/Turabian StyleHaist, Maximilian, Henner Stege, Verena Maikranz, Maria Halley Blanco, Stephan Grabbe, and Carmen Loquai. 2022. "Poliosis Is Associated with Response to Checkpoint-Inhibitor Therapy: A Case Report of Two Patients with Multifocal Metastatic Melanoma" Immuno 2, no. 2: 307-316. https://doi.org/10.3390/immuno2020020
APA StyleHaist, M., Stege, H., Maikranz, V., Halley Blanco, M., Grabbe, S., & Loquai, C. (2022). Poliosis Is Associated with Response to Checkpoint-Inhibitor Therapy: A Case Report of Two Patients with Multifocal Metastatic Melanoma. Immuno, 2(2), 307-316. https://doi.org/10.3390/immuno2020020







