Role of Inflammatory Mediators in Chronic Obstructive Pulmonary Disease Pathogenesis: Updates and Perspectives
Abstract
1. Introduction
2. Factors Influencing the Inflammatory Response in COPD
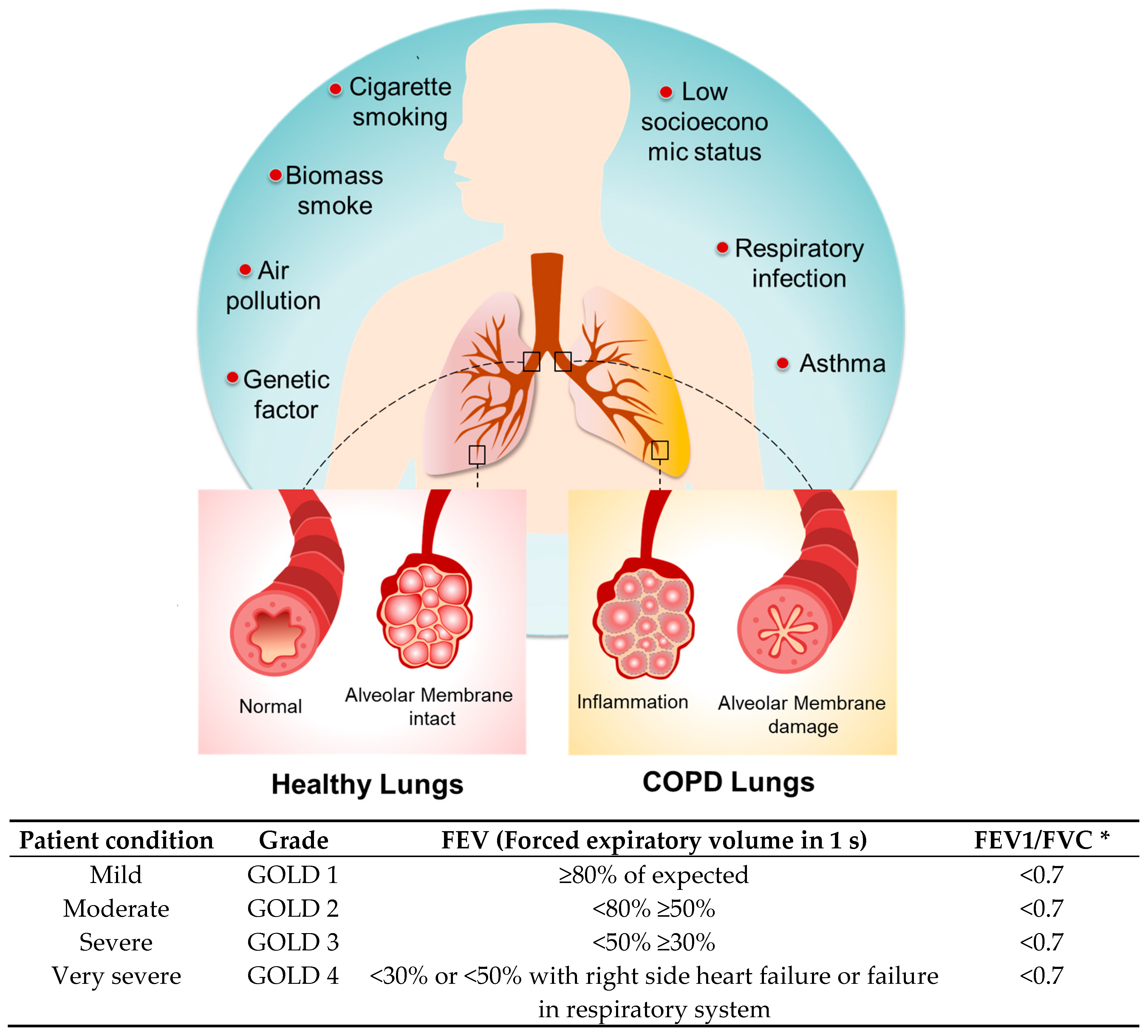
2.1. Cigarette Smoking
2.2. Genetic Factors
2.3. Accelerated Aging
2.4. Air Pollution
2.5. Respiratory Infection
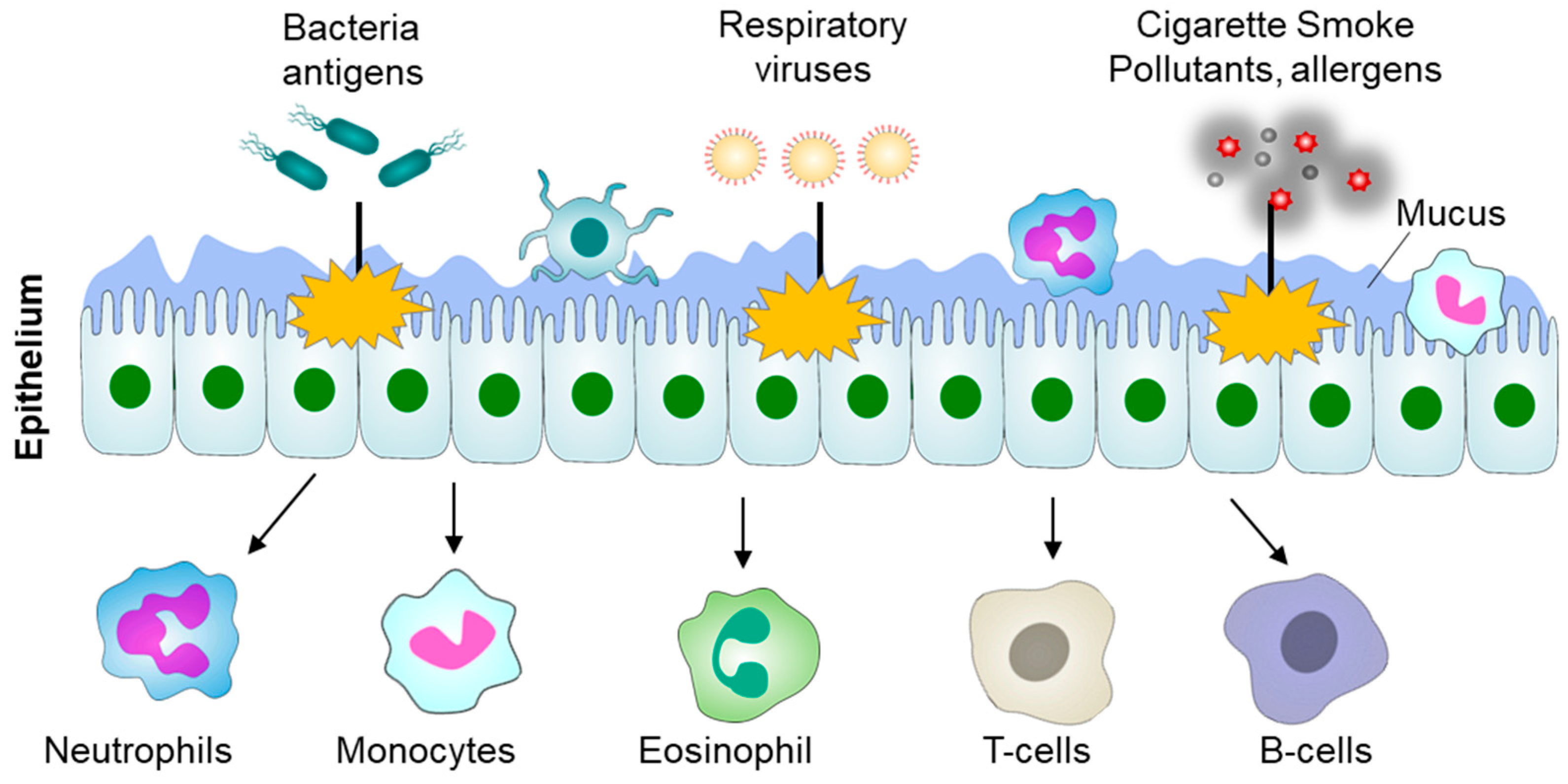
3. Role of Immune Cells in COPD
3.1. Neutrophils
3.2. Macrophages
3.3. Monocytes
3.4. Eosinophils
3.5. T-Lymphocytes
3.6. B-Lymphocytes
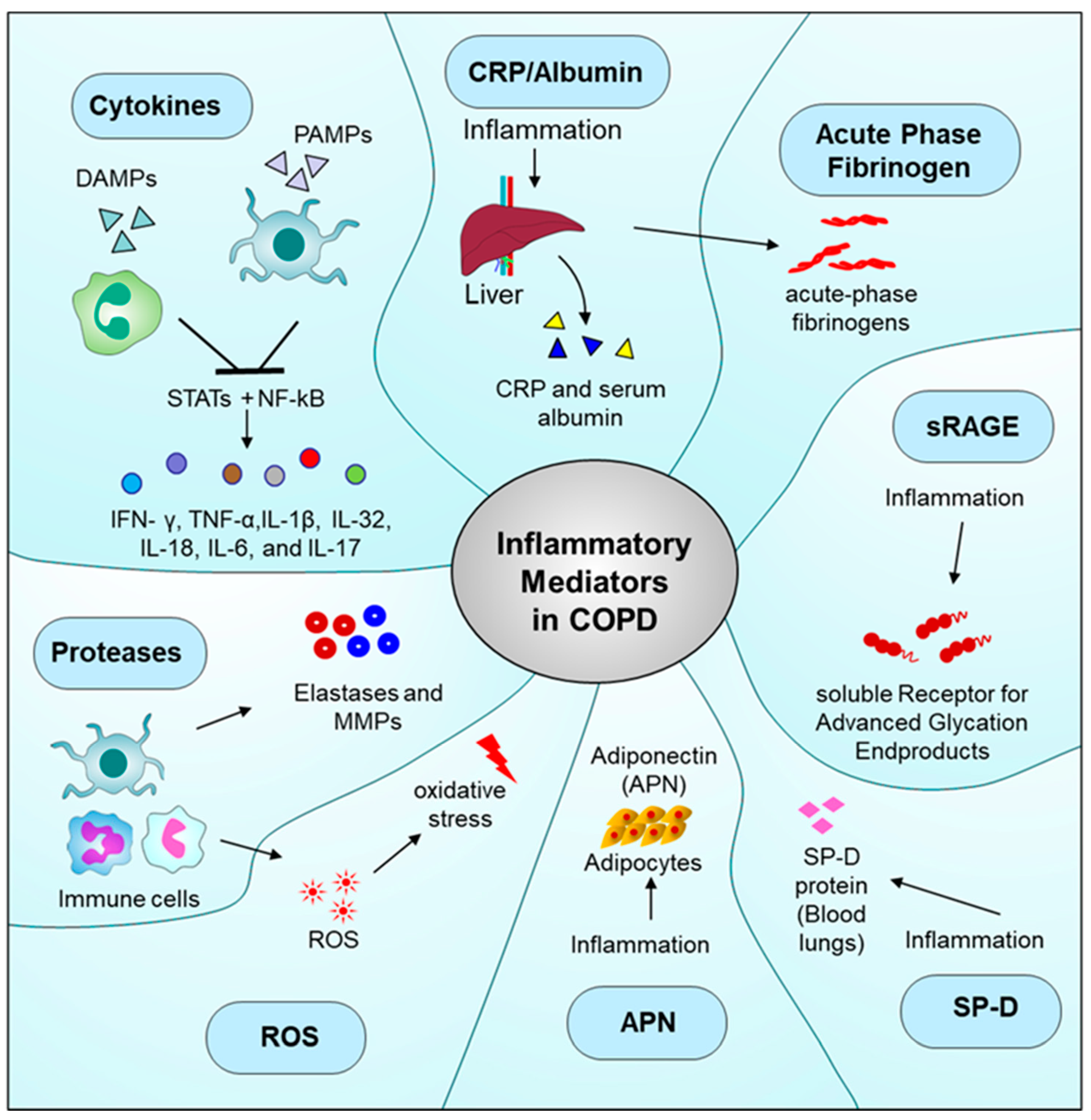
4. Inflammatory Mediators in COPD
4.1. Cytokines
4.2. C-Reactive Protein (CRP)
4.3. Fibrinogen
4.4. sRAGE
4.5. CC16
4.6. Surfactant Protein D (SP-D)
4.7. Adiponectin (APN)
4.8. ROS
4.9. Proteases
4.10. Antioxidant Defense Mechanisms
4.11. Categorization of Biomarkers in COPD
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stolz, D.; Mkorombindo, T.; Schumann, D.M.; Agusti, A.; Ash, S.Y.; Bafadhel, M.; Bai, C.; Chalmers, J.D.; Criner, G.J.; Dharmage, S.C. Towards the Elimination of Chronic Obstructive Pulmonary Disease: A Lancet Commission. Lancet 2022, 400, 921–972. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Ding, P.; Ma, J.; Yang, N.; Zheng, J.; Zhou, N. Cost-Effectiveness Analysis of the TCM “Yupingfeng Granules” in the Treatment of Acute Exacerbations of COPD Based on a Randomized Clinical Trial. Int. J. Chronic Obstr. Pulm. Dis. 2022, 17, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Zinellu, A.; Mangoni, A.A. Arginine, Transsulfuration, and Folic Acid Pathway Metabolomics in Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis. Cells 2023, 12, 2180. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Kusunose, M.; Shibayama, A.; Nakayasu, K. Comparison of Disease Severity Classifications of Chronic Obstructive Pulmonary Disease: GOLD vs. STAR in Clinical Practice. Diagnostics 2024, 14, 646. [Google Scholar] [CrossRef]
- Sayers, I.; John, C.; Chen, J.; Hall, I.P. Genetics of Chronic Respiratory Disease. Nat. Rev. Genet. 2024, 25, 534–547. [Google Scholar] [CrossRef]
- Tirelli, C.; Mira, S.; Belmonte, L.A.; De Filippi, F.; De Grassi, M.; Italia, M.; Maggioni, S.; Guido, G.; Mondoni, M.; Canonica, G.W.; et al. Exploring the Potential Role of Metabolomics in COPD: A Concise Review. Cells 2024, 13, 475. [Google Scholar] [CrossRef]
- Rodrigues, S.d.O.; da Cunha, C.M.C.; Soares, G.M.V.; Silva, P.L.; Silva, A.R.; Gonçalves-De-albuquerque, C.F. Mechanisms, Pathophysiology and Currently Proposed Treatments of Chronic Obstructive Pulmonary Disease. Pharmaceuticals 2021, 14, 979. [Google Scholar] [CrossRef]
- Calzetta, L.; Di Daniele, N.; Chetta, A.; Vitale, M.; Gholamalishahi, S.; Cazzola, M.; Rogliani, P. The Impact of Thermal Water in Asthma and COPD: A Systematic Review According to the PRISMA Statement. J. Clin. Med. 2024, 13, 1071. [Google Scholar] [CrossRef]
- Tsutsumi, A.; Chubachi, S.; Irie, H.; Sasaki, M.; Yamada, Y.; Sugiura, H.; Jinzaki, M.; Nakamura, H.; Asano, K.; Betsuyaku, T.; et al. Characteristics of Chronic Obstructive Pulmonary Disease Patients with Robust Progression of Emphysematous Change. Sci. Rep. 2021, 11, 9548. [Google Scholar] [CrossRef]
- Fuhlbrigge, A.L. Epidemiology of Asthma-Chronic Obstructive Pulmonary Disease Overlap. Immunol. Allergy Clin. 2022, 42, 533–547. [Google Scholar] [CrossRef]
- Gómez, F.P.; Rodriguez-Roisin, R. Global Initiative for Chronic Obstructive Lung Disease (GOLD) Guidelines for Chronic Obstructive Pulmonary Disease. Curr. Opin. Pulm. Med. 2002, 8, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Duffy, S.P.; Criner, G.J. Chronic Obstructive Pulmonary Disease: Evaluation and Management. Med. Clin. N. Am. 2019, 103, 453–461. [Google Scholar] [CrossRef]
- Jiang, X.-Q.; Mei, X.-D.; Feng, D. Air Pollution and Chronic Airway Diseases: What Should People Know and Do? J. Thorac. Dis. 2016, 8, E31–E40. [Google Scholar] [PubMed]
- Kotla, N.K.; Dutta, P.; Parimi, S.; Das, N.K. The Role of Ferritin in Health and Disease: Recent Advances and Understandings. Metabolites 2022, 12, 609. [Google Scholar] [CrossRef] [PubMed]
- Kotlyarov, S. Involvement of the Innate Immune System in the Pathogenesis of Chronic Obstructive Pulmonary Disease. Int. J. Mol. Sci. 2022, 23, 985. [Google Scholar] [CrossRef]
- Brightling, C.; Greening, N. Airway Inflammation in COPD: Progress to Precision Medicine. Eur. Respir. J. 2019, 54, 1900651. [Google Scholar] [CrossRef]
- Baines, K.J.; Negewo, N.A.; Gibson, P.G.; Fu, J.-J.; Simpson, J.L.; Wark, P.A.B.; Fricker, M.; McDonald, V.M. A Sputum 6 Gene Expression Signature Predicts Inflammatory Phenotypes and Future Exacerbations of COPD. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 1577–1590. [Google Scholar] [CrossRef]
- Shi, J.; Li, J. Neutrophil-Targeted Engineered Prodrug Nanoparticles for Anti-Inflammation. FASEB J. 2020, 34, 9828–9831. [Google Scholar] [CrossRef]
- Barnes, P.J. Targeting Cytokines to Treat Asthma and Chronic Obstructive Pulmonary Disease. Nat. Rev. Immunol. 2018, 18, 454–466. [Google Scholar] [CrossRef]
- Hogg, J.C.; Paré, P.D.; Hackett, T.-L. The Contribution of Small Airway Obstruction to the Pathogenesis of Chronic Obstructive Pulmonary Disease. Physiol. Rev. 2017, 97, 529–552. [Google Scholar] [CrossRef]
- Caramori, G.; Adcock, I.M.; Di Stefano, A.; Chung, K.F. Cytokine Inhibition in the Treatment of COPD. Int. J. Chronic Obstr. Pulm. Dis. 2014, 9, 397–412. [Google Scholar]
- Aghasafari, P.; George, U.; Pidaparti, R. A Review of Inflammatory Mechanism in Airway Diseases. Inflamm. Res. 2019, 68, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Arango Duque, G.; Descoteaux, A. Macrophage Cytokines: Involvement in Immunity and Infectious Diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef] [PubMed]
- Ponce-Gallegos, M.A.; Ramírez-Venegas, A.; Falfán-Valencia, R. Th17 Profile in COPD Exacerbations. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 1857–1865. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef]
- Morris, R.; Kershaw, N.J.; Babon, J.J. The Molecular Details of Cytokine Signaling via the JAK/STAT Pathway. Protein Sci. 2018, 27, 1984–2009. [Google Scholar] [CrossRef]
- Lee, Y.-G.; Lee, P.-H.; Choi, S.-M.; An, M.-H.; Jang, A.-S. Effects of Air Pollutants on Airway Diseases. Int. J. Environ. Res. Public Health 2021, 18, 9905. [Google Scholar] [CrossRef]
- Soriano, J.B.; Polverino, F.; Cosio, B.G. What Is Early COPD and Why Is It Important? Eur. Respir. J. 2018, 52, 1801448. [Google Scholar] [CrossRef]
- Mirza, S.; Clay, R.D.; Koslow, M.A.; Scanlon, P.D. COPD Guidelines: A Review of the 2018 GOLD Report. Mayo Clin. Proc. 2018, 93, 1488–1502. [Google Scholar] [CrossRef]
- Jafari, A.; Rajabi, A.; Gholian-Aval, M.; Peyman, N.; Mahdizadeh, M.; Tehrani, H. National, Regional, and Global Prevalence of Cigarette Smoking among Women/Females in the General Population: A Systematic Review and Meta-Analysis. Environ. Health Prev. Med. 2021, 26, 5. [Google Scholar] [CrossRef]
- Gupta, S.; Panchal, P.; Sadatsafavi, M.; Ghanouni, P.; Sin, D.; Pakhale, S.; To, T.; Zafari, Z.; Nimmon, L. A Personalized Biomedical Risk Assessment Infographic for People Who Smoke with COPD: A Qualitative Study. Addict. Sci. Clin. Pract. 2022, 17, 1. [Google Scholar] [CrossRef] [PubMed]
- Taucher, E.; Mykoliuk, I.; Lindenmann, J.; Smolle-Juettner, F.-M. Implications of the Immune Landscape in COPD and Lung Cancer: Smoking Versus Other Causes. Front. Immunol. 2022, 13, 846605. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.-D.; Zhang, X.-R.; Zhang, A.; Li, Z.-H.; Liu, D.; Zhang, Y.-J.; Mao, C. Associations of Genetic Risk and Smoking with Incident COPD. Eur. Respir. J. 2022, 59, 2101320. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, A.; Bade, G.; Madan, K.; Ahmed Bhat, M.; Guleria, R.; Talwar, A. Effect of Smoking and Its Cessation on the Transcript Profile of Peripheral Monocytes in COPD Patients. Int. J. Chronic Obstr. Pulm. Dis. 2022, 17, 65–77. [Google Scholar] [CrossRef]
- Frederiksen, A.L.; Laustsen, B.H.; Bælum, J.; Pedersen, M.L.; Bønløkke, J.H. Prevalence of Chronic Obstructive Pulmonary Disease and Chronic Bronchitis Among Predominantly Smoking Workers in the Seafood Industry in Greenland. Int. J. Chronic Obstr. Pulm. Dis. 2022, 17, 1167–1177. [Google Scholar] [CrossRef]
- Fahy, J.V.; Locksley, R.M. Immune Treatment Tackles a Lung Disease in Smoker. Nature 2023, 621, 476–477. [Google Scholar] [CrossRef]
- Puchakayala, P.R.A.; Sthanam, V.L.; Nakhmani, A.; Chaudhary, M.F.A.; Puliyakote, A.K.; Reinhardt, J.M.; Zhang, C.; Bhatt, S.P.; Bodduluri, S. Radiomics for Improved Detection of Chronic Obstructive Pulmonary Disease in Low-Dose and Standard-Dose Chest CT Scans. Radiology 2023, 307, 5. [Google Scholar] [CrossRef]
- Rahi, M.S.; Mudgal, M.; Asokar, B.K.; Yella, P.R.; Gunasekaran, K. Management of Refractory Chronic Obstructive Pulmonary Disease: A Review. Life 2024, 14, 542. [Google Scholar] [CrossRef]
- Wang, L.; Koelink, P.J.; Garssen, J.; Folkerts, G.; Henricks, P.A.J.; Braber, S. Gut Microbiome and Transcriptomic Changes in Cigarette Smoke-Exposed Mice Compared to COPD and CD Patient Datasets. Int. J. Mol. Sci. 2024, 25, 4058. [Google Scholar] [CrossRef]
- Zhang, J.; Wurzel, D.F.; Perret, J.L.; Lodge, C.J.; Walters, E.H.; Dharmage, S.C. Chronic Bronchitis in Children and Adults: Definitions, Pathophysiology, Prevalence, Risk Factors, and Consequences. J. Clin. Med. 2024, 13, 2413. [Google Scholar] [CrossRef]
- Strange, C. Alpha-1 Antitrypsin Deficiency Associated COPD. Clin. Chest Med. 2020, 41, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Torres-Durán, M.; Lopez-Campos, J.L.; Barrecheguren, M.; Miravitlles, M.; Martinez-Delgado, B.; Castillo, S.; Escribano, A.; Baloira, A.; Navarro-Garcia, M.M.; Pellicer, D.; et al. Alpha-1 Antitrypsin Deficiency: Outstanding Questions and Future Directions. Orphanet J. Rare Dis. 2018, 13, 114. [Google Scholar] [CrossRef] [PubMed]
- Lackey, L.; Coria, A.; Ghosh, A.J.; Grayeski, P.; Hatfield, A.; Shankar, V.; Platig, J.; Xu, Z.; Ramos, S.B.V.; Silverman, E.K.; et al. Alternative Poly-Adenylation Modulates A1-Antitrypsin Expression in Chronic Obstructive Pulmonary Disease. PLoS Genet. 2021, 17, e1009912. [Google Scholar] [CrossRef] [PubMed]
- Köhnlein, T.; Welte, T. Alpha-1 Antitrypsin Deficiency: Pathogenesis, Clinical Presentation, Diagnosis, and Treatment. Am. J. Med. 2008, 121, 3–9. [Google Scholar] [CrossRef]
- Nigro, E.; Mosella, M.; Daniele, A.; Mallardo, M.; Accardo, M.; Bianco, A.; Perrotta, F.; Scialò, F. Adiponectin Increase in Patients Affected by Chronic Obstructive Pulmonary Disease with Overlap of Bronchiectasis. Life 2023, 13, 444. [Google Scholar] [CrossRef]
- Zailani, H.; Satyanarayanan, S.K.; Liao, W.-C.; Liao, H.-F.; Huang, S.-Y.; Gałecki, P.; Su, K.-P.; Chang, J.P.-C. Omega-3 Polyunsaturated Fatty Acids in Managing Comorbid Mood Disorders in Chronic Obstructive Pulmonary Disease (COPD): A Review. J. Clin. Med. 2023, 12, 2653. [Google Scholar] [CrossRef]
- Cho, E.E.; Mecredy, G.C.; Wong, H.H.; Stanbrook, M.B.; Gershon, A.S. Which Physicians Are Taking Care of People with COPD? Chest 2019, 155, 771–777. [Google Scholar] [CrossRef]
- Paschalaki, K.E.; Starke, R.D.; Hu, Y.; Mercado, N.; Margariti, A.; Gorgoulis, V.G.; Randi, A.M.; Barnes, P.J. Dysfunction of Endothelial Progenitor Cells from Smokers and Chronic Obstructive Pulmonary Disease Patients Due to Increased DNA Damage and Senescence. Stem Cells 2013, 31, 2813–2826. [Google Scholar] [CrossRef]
- Zhong, S.; Yang, L.; Liu, N.; Zhou, G.; Hu, Z.; Chen, C.; Wang, Y. Identification and Validation of Aging-Related Genes in COPD Based on Bioinformatics Analysis. Aging 2022, 14, 4336–4356. [Google Scholar] [CrossRef]
- Al Bitar, S.; Gali-Muhtasib, H. The Role of the Cyclin Dependent Kinase Inhibitor P21cip1/Waf1 in Targeting Cancer: Molecular Mechanisms and Novel Therapeutics. Cancers 2019, 11, 1475. [Google Scholar] [CrossRef]
- Wang, L.; Tang, Y.; Chen, Y. HIF1A Gene Rs10873142 Polymorphism Is Associated with Risk of Chronic Obstructive Pulmonary Disease in a Chinese Han Population: A Case-Control Study. Biosci. Rep. 2018, 38, BSR20171309. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.-Q.; Hurlin, P.J. The Interplay between Mad and Myc in Proliferation and Differentiation. Trends Cell Biol. 2001, 11, S10–S14. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Arunachalam, G.; Hwang, J.; Chung, S.; Sundar, I.K.; Kinnula, V.L.; Crapo, J.D.; Rahman, I. Extracellular Superoxide Dismutase Protects against Pulmonary Emphysema by Attenuating Oxidative Fragmentation of ECM. Proc. Natl. Acad. Sci. USA 2010, 107, 15571–15576. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Rogliani, P.; Ora, J.; Calzetta, L.; Lauro, D.; Matera, M.G. Hyperglycaemia and Chronic Obstructive Pulmonary Disease. Diagnostics 2023, 13, 3362. [Google Scholar] [CrossRef]
- Elkhapery, A.; Hammami, M.B.; Sulica, R.; Boppana, H.; Abdalla, Z.; Iyer, C.; Taifour, H.; Niu, C.; Deshwal, H. Pulmonary Vasodilator Therapy in Severe Pulmonary Hypertension Due to Chronic Obstructive Pulmonary Disease (Severe PH-COPD): A Systematic Review and Meta-Analysis. J. Cardiovasc. Dev. Dis. 2023, 10, 498. [Google Scholar] [CrossRef]
- He, Y.; Qian, D.C.; Diao, J.A.; Cho, M.C.; Silverman, E.K.; Gusev, A.; Manrai, A.K.; Martin, A.R.; Patel, C.J. Prediction and Stratification of Longitudinal Risk for Chronic Obstructive Pulmonary Disease Across Smoking Behaviors. Nat. Commun. 2023, 14, 8297. [Google Scholar] [CrossRef]
- Aghapour, M.; Ubags, N.D.; Bruder, D.; Hiemstra, P.S.; Sidhaye, V.; Rezaee, F.; Heijink, I.H. Role of Air Pollutants in Airway Epithelial Barrier Dysfunction in Asthma and COPD. Eur. Respir. Rev. 2022, 31, 210112. [Google Scholar] [CrossRef]
- Huh, J.-Y.; Hong, J.; Han, D.-W.; Park, Y.-J.; Jung, J.; Lee, S.W. The Impact of Air Pollutants and Meteorological Factors on Chronic Obstructive Pulmonary Disease Exacerbations: A Nationwide Study. Ann. Am. Thorac. Soc. 2022, 19, 214–226. [Google Scholar] [CrossRef]
- Kazemiparkouhi, F.; Eum, K.-D.; Wang, B.; Manjourides, J.; Suh, H.H. Long-Term Ozone Exposures and Cause-Specific Mortality in a US Medicare Cohort. J. Expo. Sci. Environ. Epidemiol. 2020, 30, 650–658. [Google Scholar] [CrossRef]
- Chen, R.; Yin, P.; Meng, X.; Wang, L.; Liu, C.; Niu, Y.; Lin, Z.; Liu, Y.; Liu, J.; Qi, J. Associations between Ambient Nitrogen Dioxide and Daily Cause-Specific Mortality: Evidence from 272 Chinese Cities. Epidemiology 2018, 29, 482–489. [Google Scholar] [CrossRef]
- Cai, J.; Peng, C.; Yu, S.; Pei, Y.; Liu, N.; Wu, Y.; Fu, Y.; Cheng, J. Association between PM2. 5 Exposure and All-Cause, Non-Accidental, Accidental, Different Respiratory Diseases, Sex and Age Mortality in Shenzhen, China. Int. J. Environ. Res. Public Health 2019, 16, 401. [Google Scholar] [CrossRef] [PubMed]
- Kariisa, M.; Foraker, R.; Pennell, M.; Buckley, T.; Diaz, P.; Criner, G.J.; Wilkins III, J.R. Short-and Long-Term Effects of Ambient Ozone and Fine Particulate Matter on the Respiratory Health of Chronic Obstructive Pulmonary Disease Subjects. Arch. Environ. Occup. Health 2015, 70, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Cobos, F.; Aguiar-Leiva, V.P.; Argüello-Suárez, C.; Colacicchi, P.; Calleja-Cartón, L.A.; Leiva-Fernández, F. Validation of an Inhaled Therapy Beliefs Questionnaire in Patients with Chronic Obstructive Pulmonary Disease. J. Clin. Med. 2024, 13, 2281. [Google Scholar] [CrossRef] [PubMed]
- Rajnoveanu, R.-M.; Harangus, A.; Todea, D.A.; Man, M.A.; Budin, C.E.; Rajnoveanu, A.-G. Opioids in Treatment of Refractory Dyspnea in Chronic Obstructive Pulmonary Disease: Yes, No or Maybe. J. Pers. Med. 2024, 14, 318. [Google Scholar] [CrossRef]
- Aegerter, H.; Lambrecht, B.N.; Jakubzick, C.V. Biology of Lung Macrophages in Health and Disease. Immunity 2022, 55, 1564–1580. [Google Scholar] [CrossRef]
- Martinez-Garcia, M.A.; Miravitlles, M. The Impact of Chronic Bronchial Infection in COPD: A Proposal for Management. Int. J. Chronic Obstr. Pulm. Dis. 2022, 17, 621–630. [Google Scholar] [CrossRef]
- Wang, Z.; Locantore, N.; Haldar, K.; Ramsheh, M.Y.; Beech, A.S.; Ma, W.; Brown, J.R.; Tal-Singer, R.; Barer, M.R.; Bafadhel, M.; et al. Inflammatory Endotype-Associated Airway Microbiome in Chronic Obstructive Pulmonary Disease Clinical Stability and Exacerbations: A Multicohort Longitudinal Analysis. Am. J. Respir. Crit. Care Med. 2021, 203, 1488–1502. [Google Scholar] [CrossRef]
- Wilson, R. The Role of Infection in COPD. Chest 1998, 113, 242S–248S. [Google Scholar] [CrossRef]
- Millares, L.; Monso, E. The Microbiome in COPD: Emerging Potential for Microbiome-Targeted Interventions. Int. J. Chronic Obstr. Pulm. Dis. 2022, 17, 1835–1845. [Google Scholar] [CrossRef]
- Tiew, P.Y.; Thng, K.X.; Chotirmall, S.H. Clinical Aspergillus Signatures in COPD and Bronchiectasis. J. Fungi 2022, 8, 480. [Google Scholar] [CrossRef]
- Singh, D.; Mathioudakis, A.G.; Higham, A. Chronic Obstructive Pulmonary Disease and COVID-19: Interrelationships. Curr. Opin. Pulm. Med. 2022, 28, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Coronado, K.; Herrada, C.; Rojas, D.A. Role of the Fungus Pneumocystis in IL1 Pathway Activation and Airways Collagen Deposition in Elastase-Induced COPD Animals. Int. J. Mol. Sci. 2024, 25, 3150. [Google Scholar] [CrossRef] [PubMed]
- Riondino, S.; Rosenfeld, R.; Formica, V.; Morelli, C.; Parisi, G.; Torino, F.; Mariotti, S.; Roselli, M. Effectiveness of Immunotherapy in Non-Small Cell Lung Cancer Patients with a Diagnosis of COPD: Is This a Hidden Prognosticator for Survival and a Risk Factor for Immune-Related Adverse Events? Cancers 2024, 16, 1251. [Google Scholar] [CrossRef] [PubMed]
- Lugg, S.T.; Scott, A.; Parekh, D.; Naidu, B.; Thickett, D.R. Cigarette Smoke Exposure and Alveolar Macrophages: Mechanisms for Lung Disease. Thorax 2022, 77, 94–101. [Google Scholar] [CrossRef]
- Poto, R.; Loffredo, S.; Palestra, F.; Marone, G.; Patella, V.; Varricchi, G. Angiogenesis, Lymphangiogenesis, and Inflammation in Chronic Obstructive Pulmonary Disease (COPD): Few Certainties and Many Outstanding Questions. Cells 2022, 11, 1720. [Google Scholar] [CrossRef]
- Lea, S.; Beech, A.; Baker, J.; Gaskell, R.; Pindolia, D.; Dikwa, A.B.; Shah, R.; Singh, D. Differential Responses of COPD Macrophages to Respiratory Bacterial Pathogens. ERJ Open Res. 2022, 8, 44–2022. [Google Scholar] [CrossRef]
- Keir, H.R.; Chalmers, J.D. Neutrophil Extracellular Traps in Chronic Lung Disease: Implications for Pathogenesis and Therapy. Eur. Respir. Rev. 2022, 31, 210241. [Google Scholar] [CrossRef]
- Ham, J.; Kim, J.; Ko, Y.G.; Kim, H.Y. The Dynamic Contribution of Neutrophils in the Chronic Respiratory Diseases. Allergy Asthma Immunol. Res. 2022, 14, 361. [Google Scholar] [CrossRef]
- Hoenderdos, K.; Condliffe, A. The Neutrophil in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Cell Mol. Biol. 2013, 48, 531–539. [Google Scholar] [CrossRef]
- Jasper, A.E.; McIver, W.J.; Sapey, E.; Walton, G.M. Understanding the Role of Neutrophils in Chronic Inflammatory Airway Disease. F1000Research 2019, 8, F1000 Faculty Rev-557. [Google Scholar] [CrossRef]
- Guo, P.; Li, R.; Piao, T.H.; Wang, C.L.; Wu, X.L.; Cai, H.Y. Pathological Mechanism and Targeted Drugs of COPD. Int. J. Chronic Obstr. Pulm. Dis. 2022, 17, 1565–1575. [Google Scholar] [CrossRef] [PubMed]
- Lodge, K.M.; Vassallo, A.; Liu, B.; Long, M.; Tong, Z.; Newby, P.R.; Agha-Jaffar, D.; Paschalaki, K.; Green, C.E.; Belchamber, K.B.R. Hypoxia Increases the Potential for Neutrophil-Mediated Endothelial Damage in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2022, 205, 903–916. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; Wang, B.; Zhong, Y.; Liu, B.; Dong, J. Modified Bushen Yiqi Formula Attenuates Neutrophils Recruitment to the Lung in Rats Model of COPD via Inhibiting the CXCL1/CXCL5/CXCL8-CXCR2 Axis and Its Downstream STAT and SRC Signaling Pathways. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Li, C.-L.; Liu, J.-F.; Liu, S.-F. Mitochondrial Dysfunction in Chronic Obstructive Pulmonary Disease: Unraveling the Molecular Nexus. Biomedicines 2024, 12, 814. [Google Scholar] [CrossRef]
- Akata, K.; Van Eeden, S.F. Lung Macrophage Functional Properties in Chronic Obstructive Pulmonary Disease. Int. J. Mol. Sci. 2020, 21, 853. [Google Scholar] [CrossRef]
- Shen, S.; Huang, Q.; Liu, L.; Zou, X.; Kang, T.; Wu, J. GATA2 Downregulation Contributes to Pro-Inflammatory Phenotype and Defective Phagocytosis of Pulmonary Macrophages in Chronic Obstructive Pulmonary Disease. Aging 2024, 16, 12928–12951. [Google Scholar] [CrossRef]
- He, S.; Tian, R.; Zhang, X.; Yao, Q.; Chen, Q.; Liu, B.; Liao, L.; Gong, Y.; Yang, Y.; Wang, D. PPARγ Inhibits Small Airway Remodeling Through Mediating The Polarization Homeostasis Of Alveolar Macrophages in COPD. Clin. Immunol. 2023, 250, 109293. [Google Scholar] [CrossRef]
- Kapellos, T.S.; Bonaguro, L.; Gemünd, I.; Reusch, N.; Saglam, A.; Hinkley, E.R.; Schultze, J.L. Human Monocyte Subsets and Phenotypes in Major Chronic Inflammatory Diseases. Front. Immunol. 2019, 10, 2035. [Google Scholar] [CrossRef]
- Burgoyne, R.A.; Fisher, A.J.; Borthwick, L.A. The Role of Epithelial Damage in the Pulmonary Immune Response. Cells 2021, 10, 2763. [Google Scholar] [CrossRef]
- Conrad, M.; Pratt, D.A. The Chemical Basis of Ferroptosis. Nat. Chem. Biol. 2019, 15, 1137–1147. [Google Scholar] [CrossRef]
- Doll, S.; Proneth, B.; Tyurina, Y.Y.; Panzilius, E.; Kobayashi, S.; Ingold, I.; Irmler, M.; Beckers, J.; Aichler, M.; Walch, A.; et al. ACSL4 Dictates Ferroptosis Sensitivity by Shaping Cellular Lipid Composition. Nat. Chem. Biol. 2017, 13, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Tőkés-Füzesi, M.; Ruzsics, I.; Rideg, O.; Kustán, P.; Kovács, G.L.; Molnár, T. Role of Microparticles Derived from Monocytes, Endothelial Cells and Platelets in the Exacerbation of COPD. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 3749–3757. [Google Scholar] [CrossRef] [PubMed]
- Rho, J.; Seo, C.-S.; Hong, E.-J.; Baek, E.B.; Jung, E.; Park, S.; Lee, M.-Y.; Kwun, H.-J. Yijin-Tang Attenuates Cigarette Smoke and Lipopolysaccharide-Induced Chronic Obstructive Pulmonary Disease in Mice. Evid. Based Complement. Altern. Med. 2022, 2022, 7902920. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Chan, H.P.; Gan, P.X.L.; Liew, M.F.; Wong, W.S.F.; Lim, H.-F. Eosinophilic Endotype of Chronic Obstructive Pulmonary Disease: Similarities and Differences from Asthma. Korean J. Intern. Med. 2021, 36, 1305–1319. [Google Scholar] [CrossRef]
- Gaur, P.; Zaffran, I.; George, T.; Rahimli Alekberli, F.; Ben-Zimra, M.; Levi-Schaffer, F. The Regulatory Role of Eosinophils in Viral, Bacterial, and Fungal Infections. Clin. Exp. Immunol. 2022, 209, 72–82. [Google Scholar] [CrossRef]
- Brusselle, G.; Pavord, I.D.; Landis, S.; Pascoe, S.; Lettis, S.; Morjaria, N.; Barnes, N.; Hilton, E. Blood Eosinophil Levels as a Biomarker in COPD. Respir. Med. 2018, 138, 21–31. [Google Scholar] [CrossRef]
- David, B.; Bafadhel, M.; Koenderman, L.; De Soyza, A. Eosinophilic Inflammation in COPD: From an Inflammatory Marker to a Treatable Trait. Thorax 2021, 76, 188–195. [Google Scholar] [CrossRef]
- Weissler, J.C.; Adams, T.N. Eosinophilic Chronic Obstructive Pulmoary Disease. Lung 2021, 199, 589–595. [Google Scholar] [CrossRef]
- Chen, K.; Kolls, J.K. T Cell-Mediated Host Immune Defenses in the Lung. Annu. Rev. Immunol. 2013, 31, 605–633. [Google Scholar] [CrossRef]
- Tam, A.; Tanabe, N.; Churg, A.; Wright, J.L.; Hogg, J.C.; Sin, D.D. Sex Differences in Lymphoid Follicles in COPD Airways. Respir. Res. 2020, 21, 46. [Google Scholar] [CrossRef]
- Paats, M.S.; Bergen, I.M.; Hoogsteden, H.C.; van der Eerden, M.M.; Hendriks, R.W. Systemic CD4+ and CD8+ T-Cell Cytokine Profiles Correlate with GOLD Stage in Stable COPD. Eur. Respir. J. 2012, 40, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, J.D.; Ito, J.T.; Martins, M.d.A.; Tibério, I.d.F.L.C.; Lopes, F.D.T.Q.D.S. Th17/Treg Imbalance in Chronic Obstructive Pulmonary Disease: Clinical and Experimental Evidence. Front. Immunol. 2021, 12, 804919. [Google Scholar] [CrossRef] [PubMed]
- Corleis, B.; Cho, J.L.; Gates, S.J.; Linder, A.H.; Dickey, A.; Lisanti-Park, A.C.; Schiff, A.E.; Ghebremichael, M.; Kohli, P.; Winkler, T.; et al. Smoking and Human Immunodeficiency Virus 1 Infection Promote Retention of CD8+ T Cells in the Airway Mucosa. Am. J. Respir. Cell Mol. Biol. 2021, 65, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Huang, K.; Yu, T.; Chang, C.; Chu, X.; Huang, Y.; Zheng, Z.; Ma, L.; Li, B.; Yang, T. Interleukin-2/Anti-Interleukin-2 Complex Attenuates Inflammation in a Mouse COPD Model by Expanding CD4+ CD25+ Foxp3+ Regulatory T Cells. Int. Immunopharmacol. 2024, 131, 111849. [Google Scholar] [CrossRef]
- Ström, J.E.; Pourazar, J.; Linder, R.; Blomberg, A.; Lindberg, A.; Bucht, A.; Behndig, A.F. Airway Regulatory T Cells are Decreased in COPD with a Rapid Decline in Lung Function. Respir. Res. 2020, 21, 330. [Google Scholar] [CrossRef]
- Silva, L.; Lourenço, J.; Silva, K.; Santana, F.; Kohler, J.; Moreira, A.; Velosa, A.P.P.; Prado, C.M.; Vieira, R.P.; Aun, M.V.; et al. Th17/Treg Imbalance in COPD Development: Suppressors of Cytokine Signaling and Signal Transducers and Activators of Transcription Proteins. Sci. Rep. 2020, 10, 15287. [Google Scholar] [CrossRef]
- Donovan, C.; Starkey, M.R.; Kim, R.Y.; Rana, B.M.J.; Barlow, J.L.; Jones, B.; Haw, T.J.; Mono Nair, P.; Budden, K.; Cameron, G.J.M.; et al. Roles for T/B Lymphocytes and ILC2s in Experimental Chronic Obstructive Pulmonary Disease. J. Leukoc. Biol. 2019, 105, 143–150. [Google Scholar] [CrossRef]
- Kadushkin, A.G.; Tahanovich, A.D.; Movchan, L.V.; Zafranskaya, M.M.; Dziadzichkina, V.V.; Shman, T.V. Population rearrangement of B-lymphocytes expressing chemokine receptors in patients with chronic obstructive pulmonary disease. Biomed. Khim. 2022, 68, 134–143. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, J.; Meng, Y.; Adcock, I.M.; Yao, X. Role of Inflammatory Cells in Airway Remodeling in COPD. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 3341–3348. [Google Scholar] [CrossRef]
- Furci, F.; Murdaca, G.; Pelaia, C.; Imbalzano, E.; Pelaia, G.; Caminati, M.; Allegra, A.; Senna, G.; Gangemi, S. TSLP and HMGB1: Inflammatory Targets and Potential Biomarkers for Precision Medicine in Asthma and COPD. Biomedicines 2023, 11, 437. [Google Scholar] [CrossRef]
- Riera-Martinez, L.; Cànaves-Gómez, L.; Iglesias, A.; Martin-Medina, A.; Cossio, B.G. The Role of IL-33/ST2 in COPD and its Future as an Antibody Therapy. Int. J. Mol. Sci. 2023, 24, 8702. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhou, X.; Ju, R.; Leng, J.; Tian, J.; Qu, S.; Tao, S.; Lv, Y.; Zhang, N. Challenges in Clinical Practice, Biological Mechanism and Prospects of Physical Ablation Therapy for COPD. Life Sci. 2024, 349, 122718. [Google Scholar] [CrossRef] [PubMed]
- Schleich, F.; Bougard, N.; Moermans, C.; Sabbe, M.; Louis, R. Cytokine-Targeted Therapies for Asthma and COPD. Eur. Respir. Rev. 2023, 32, 220193. [Google Scholar] [CrossRef] [PubMed]
- Vanfleteren, L.E.G.W.; Weidner, J.; Franssen, F.M.E.; Gaffron, S.; Reynaert, N.L.; Wouters, E.F.M.; Spruit, M.A. Biomarker-Based Clustering of Patients with Chronic Obstructive Pulmonary Disease. ERJ Open Res. 2023, 9, 00301–02022. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, T.; Wang, J.; Cheng, Y.; Zeng, Q.; You, Z.; Dai, G. Analysis of Network Expression and Immune Infiltration of Disulfidptosis-Related Genes in Chronic Obstructive Pulmonary Disease. Immun. Inflamm. Dis. 2024, 12, e1231. [Google Scholar] [CrossRef]
- Yehia, D.; Leung, C.; Sin, D.D. Clinical Utilization of Airway Inflammatory Biomarkers in the Prediction and Monitoring of Clinical Outcomes in Patients with Chronic Obstructive Pulmonary Disease. Expert Rev. Mol. Diagn. 2024, 24, 409–421. [Google Scholar] [CrossRef]
- Lacy, P.; Stow, J.L. Cytokine Release from Innate Immune Cells: Association with Diverse Membrane Trafficking Pathways. Blood 2011, 118, 9–18. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; Kagan, J.C. Toll-like Receptors and the Control of Immunity. Cell 2020, 180, 1044–1066. [Google Scholar] [CrossRef]
- Anthoney, N.; Foldi, I.; Hidalgo, A. Toll and Toll-like Receptor Signalling in Development. Development 2018, 145, dev.156018. [Google Scholar] [CrossRef]
- Alharbi, K.S.; Fuloria, N.K.; Fuloria, S.; Rahman, S.B.; Al-Malki, W.H.; Javed Shaikh, M.A.; Thangavelu, L.; Singh, S.K.; Rama Raju Allam, V.S.; Jha, N.K.; et al. Nuclear Factor-Kappa B and Its Role in Inflammatory Lung Disease. Chem. Biol. Interact. 2021, 345, 109568. [Google Scholar] [CrossRef]
- Chen, G.; Mu, Q.; Meng, Z.-J. Cigarette Smoking Contributes to Th1/Th2 Cell Dysfunction via the Cytokine Milieu in Chronic Obstructive Pulmonary Disease. Int. J. Chronic Obstr. Pulm. Dis. 2023, 18, 2027–2038. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.; Asrat, S.; Allinne, J.; Lim, W.K.; Nagashima, K.; Birchard, D.; Srivatsan, S.; Ajithdoss, D.K.; Oyejide, A.; Ben, L.-H.; et al. IL-4 and IL-13.; not Eosinophils, Drive Type 2 Airway Inflammation, Remodeling and Lung Function Decline. Cytokine 2022, 162, 156091. [Google Scholar] [CrossRef] [PubMed]
- Crawford, M.; Sinha, S.; Renavikar, P.; Borcherding, N.; Karandikar, N. CD4 T Cell-Intrinsic Role for the T Helper 17 Signature Cytokine IL-17: Effector Resistance to Immune Suppression. Proc. Natl. Acad. Sci. USA 2020, 117, 19408–19414. [Google Scholar] [CrossRef] [PubMed]
- Shyam Prasad Shetty, B.; Chaya, S.K.; Kumar, V.S.; Mahendra, M.; Jayaraj, B.S.; Lokesh, K.S.; Ganguly, K.; Mahesh, P.A. Inflammatory Biomarkers Interleukin 1 Beta (IL-1β) and Tumour Necrosis Factor Alpha (TNF-α) Are Differentially Elevated in Tobacco Smoke Associated COPD and Biomass Smoke Associated COPD. Toxics 2021, 9, 9040072. [Google Scholar] [CrossRef]
- De Rubis, G.; Paudel, K.; Manandhar, B.; Singh, S.K.; Gupta, G.; Malik, R.; Shen, J.; Chami, A.; MacLoughlin, R.; Chellappan, D.K.; et al. Agarwood Oil Nanoemulsion Attenuates Cigarette Smoke-Induced Inflammation and Oxidative Stress Markers in BCi-NS1.1 Airway Epithelial Cells. Nutrients 2023, 15, 1019. [Google Scholar] [CrossRef]
- Parameswaran, N.; Patial, S. Tumor Necrosis Factor-α Signaling in Macrophages. Crit. Rev. Eukaryot. Gene Expr. 2010, 20, 87–103. [Google Scholar] [CrossRef]
- Mir, H.; Koul, P.A.; Bhat, D.; Shah, Z.A. A Case-Control Study of Tumor Necrosis Factor-Alpha Promoter Polymorphism and Its Serum Levels in Patients with Chronic Obstructive Pulmonary Disease in Kashmir, North India. Lung India 2020, 37, 204–209. [Google Scholar]
- Yao, Y.; Zhou, J.; Diao, X.; Wang, S. Association between Tumor Necrosis Factor-α and Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis. Ther. Adv. Respir. Dis. 2019, 13, 1753466619866096. [Google Scholar] [CrossRef]
- Mantovani, A.; Dinarello, C.A.; Molgora, M.; Garlanda, C. Interleukin-1 and Related Cytokines in the Regulation of Inflammation and Immunity. Immunity 2019, 50, 778–795. [Google Scholar] [CrossRef]
- Lin, X.; Fan, Y.; Wang, X.; Chi, M.; Li, X.; Zhang, X.; Sun, D. Correlation Between Tumor Necrosis Factor-α and Interleukin-1β in Exhaled Breath Condensate and Pulmonary Function. Am. J. Med. Sci. 2017, 354, 388–394. [Google Scholar] [CrossRef]
- Feng, Q.; Yu, Y.-Z.; Meng, Q.-H. Blocking Tumor Necrosis Factor-α Delays Progression of Chronic Obstructive Pulmonary Disease in Rats through Inhibiting MAPK Signaling Pathway and Activating SOCS3/TRAF1. Exp. Ther. Med. 2021, 22, 1311. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Feng, D.; Dong, S.; He, R.; Fan, B. Dysregulated Circulating MicroRNA-126 in Chronic Obstructive Pulmonary Disease: Linkage with Acute Exacerbation Risk, Severity Degree, and Inflammatory Cytokines. J. Clin. Lab. Anal. 2022, 36, e24204. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-Y.; Lin, Y.-M.; Wu, J.-H.; Zhang, X.-Q.; Zhang, Y.; Xie, W.-X.; Chu, S.-Q.; Li, Y. Effect of Doxofylline on Pulmonary Inflammatory Response and Oxidative Stress during Mechanical Ventilation in Rats with COPD. BMC Pulm. Med. 2022, 22, 66. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Li, X.; Fang, N.; Li, P.; Zhang, Z.; Lin, M.; Hou, Q. Perilla Leaf Extract (PLE) Attenuates COPD Airway Inflammation via the TLR4/Syk/PKC/NF-ΚB Pathway In Vivo and In Vitro. Front. Pharmacol. 2021, 12, 763624. [Google Scholar] [CrossRef]
- Zhang, C.; Yuan, Y.; Song, J.; Li, X.; Wang, J. Elevated Serum Myeloid-Related Protein (MRP) 8/14 in Patients with Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Clin. Lab. 2021, 67, 1. [Google Scholar] [CrossRef]
- Anderson, G.P. COPD, Asthma and C-Reactive Protein. Eur. Respir. J. 2006, 27, 874–876. [Google Scholar] [CrossRef]
- de Torres, J.P.; Cordoba-Lanus, E.; López-Aguilar, C.; Muros de Fuentes, M.; Montejo de Garcini, A.; Aguirre-Jaime, A.; Celli, B.R.; Casanova, C. C-Reactive Protein Levels and Clinically Important Predictive Outcomes in Stable COPD Patients. Eur. Respir. J. 2006, 27, 902–907. [Google Scholar] [CrossRef]
- Baldemir, R.; Öztürk, A.; Eraslan Doganay, G.; Cirik, M.O.; Alagoz, A. Evaluation of Nutritional Status in Hospitalized Chronic Obstructive Pulmonary Disease Patients and Can C-Reactive Protein-to-Albumin Ratio Be Used in the Nutritional Risk Assessment in These Patients. Cureus 2022, 14, e21833. [Google Scholar] [CrossRef]
- Yang, D.; Wang, L.; Jiang, P.; Kang, R.; Xie, Y. Correlation between Hs-CRP, IL-6, IL-10, ET-1, and Chronic Obstructive Pulmonary Disease Combined with Pulmonary Hypertension. J. Healthc. Eng. 2022, 2022, 3247807. [Google Scholar] [CrossRef]
- Wang, H.; Qu, G. Observation of the Effect of Singulair Combined With Ketotifen in the Treatment of Acute Exacerbation of Chronic Obstructive Pulmonary Disease With Airway Hyperresponsiveness and Its Influence on Th17/Treg. Front. Surg. 2022, 9, 848724. [Google Scholar] [CrossRef]
- Singh, R.; Narang, M.; Dawson, L.; Kamra, N.; Singh, G.; Bahamania, K.K. Could Disease Severity and Inflammatory Markers (IL-6, Hs-CRP, TNF-α) Be Related to Frailty in COPD? A Prospective Study. J. Assoc. Physicians India 2022, 70, 11–12. [Google Scholar] [PubMed]
- Miller, B.E.; Tal-Singer, R.; Rennard, S.I.; Furtwaengler, A.; Leidy, N.; Lowings, M.; Martin, U.J.; Martin, T.R.; Merrill, D.D.; Snyder, J.; et al. Plasma Fibrinogen Qualification as a Drug Development Tool in Chronic Obstructive Pulmonary Disease. Perspective of the Chronic Obstructive Pulmonary Disease Biomarker Qualification Consortium. Am. J. Respir. Crit. Care Med. 2016, 193, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Luyendyk, J.P.; Schoenecker, J.G.; Flick, M.J. The Multifaceted Role of Fibrinogen in Tissue Injury and Inflammation. Blood 2019, 133, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Pieters, M.; Wolberg, A.S. Fibrinogen and Fibrin: An Illustrated Review. Res. Pract. Thromb. Haemost. 2019, 3, 161–172. [Google Scholar] [CrossRef]
- Sparvero, L.J.; Asafu-Adjei, D.; Kang, R.; Tang, D.; Amin, N.; Im, J.; Rutledge, R.; Lin, B.; Amoscato, A.A.; Zeh, H.J.; et al. RAGE (Receptor for Advanced Glycation Endproducts), RAGE Ligands, and Their Role in Cancer and Inflammation. J. Transl. Med. 2009, 7, 17. [Google Scholar] [CrossRef]
- Wang, J.M.; Han, M.K.; Labaki, W.W. Chronic Obstructive Pulmonary Disease Risk Assessment Tools: Is One Better than the Others? Curr. Opin. Pulm. Med. 2022, 28, 99–108. [Google Scholar] [CrossRef]
- Pratte, K.A.; Curtis, J.L.; Kechris, K.; Couper, D.; Cho, M.H.; Silverman, E.K.; DeMeo, D.L.; Sciurba, F.C.; Zhang, Y.; Ortega, V.E.; et al. Soluble Receptor for Advanced Glycation End Products (SRAGE) as a Biomarker of COPD. Respir. Res. 2021, 22, 127. [Google Scholar] [CrossRef]
- Almuntashiri, S.; Zhu, Y.; Han, Y.; Wang, X.; Somanath, P.R.; Zhang, D. Club Cell Secreted Protein CC16: Potential Applications in Prognosis and Therapy for Pulmonary Diseases. J. Clin. Med. 2020, 9, 4039. [Google Scholar] [CrossRef]
- Chukowry, P.S.; Spittle, D.A.; Turner, A.M. Small Airways Disease, Biomarkers and COPD: Where Are We? Int. J. Chronic Obstr. Pulm. Dis. 2021, 16, 351–365. [Google Scholar] [CrossRef]
- Rong, B.; Fu, T.; Gao, W.; Li, M.; Rong, C.; Liu, W.; Liu, H. Reduced Serum Concentration of CC16 Is Associated with Severity of Chronic Obstructive Pulmonary Disease and Contributes to the Diagnosis and Assessment of the Disease. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 461–470. [Google Scholar]
- Sorensen, G.L. Surfactant Protein D in Respiratory and Non-Respiratory Diseases. Front. Med. 2018, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Zien Alaabden, A.; Mohammad, Y.; Fahoum, S. The Role of Serum Surfactant Protein D as a Biomarker of Exacerbation of Chronic Obstructive Pulmonary Disease. Qatar Med. J. 2015, 2015, 18. [Google Scholar] [CrossRef] [PubMed]
- Palma, G.; Sorice, G.P.; Genchi, V.A.; Giordano, F.; Caccioppoli, C.; D’Oria, R.; Marrano, N.; Biondi, G.; Giorgino, F.; Perrini, S. Adipose Tissue Inflammation and Pulmonary Dysfunction in Obesity. Int. J. Mol. Sci. 2022, 23, 7349. [Google Scholar] [CrossRef] [PubMed]
- Jaswal, S.; Saini, V.; Kaur, J.; Gupta, S.; Kaur, H.; Garg, K. Association of Adiponectin with Lung Function Impairment and Disease Severity in Chronic Obstructive Pulmonary Disease. Int. J. Appl. Basic Med. Res. 2018, 8, 14–18. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Jiang, T.-X.; Hu, S.-X.; Shi, Y.-H. Association between Serum Adiponectin Concentrations and Chronic Obstructive Pulmonary Disease: A Meta-Analysis. Biosci. Rep. 2020, 40, BSR20192234. [Google Scholar] [CrossRef]
- McGuinness, A.J.A.; Sapey, E. Oxidative Stress in COPD: Sources, Markers, and Potential Mechanisms. J. Clin. Med. 2017, 6, 21. [Google Scholar] [CrossRef]
- Marginean, C.; Popescu, M.S.; Vladaia, M.; Tudorascu, D.; Pirvu, D.C.; Petrescu, F. Involvement of Oxidative Stress in COPD. Curr. Health Sci. J. 2018, 44, 48–55. [Google Scholar]
- Barnes, P.J. Oxidative Stress-Based Therapeutics in COPD. Redox Biol. 2020, 33, 101544. [Google Scholar] [CrossRef]
- Sauler, M.; Lamontagne, M.; Finnemore, E.; Herazo-Maya, J.D.; Tedrow, J.; Zhang, X.; Morneau, J.E.; Sciurba, F.; Timens, W.; Paré, P.D.; et al. The DNA Repair Transcriptome in Severe COPD. Eur. Respir. J. 2018, 52, 1701994. [Google Scholar] [CrossRef]
- Taniguchi, A.; Tsuge, M.; Miyahara, N.; Tsukahara, H. Reactive Oxygen Species and Antioxidative Defense in Chronic Obstructive Pulmonary Disease. Antioxidants 2021, 10, 1537. [Google Scholar] [CrossRef]
- Gharib, S.A.; Manicone, A.M.; Parks, W.C. Matrix Metalloproteinases in Emphysema. Matrix Biol. 2018, 73, 34–51. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; Jaspers, I. Respiratory Protease/Antiprotease Balance Determines Susceptibility to Viral Infection and Can Be Modified by Nutritional Antioxidants. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 308, L1189–L1201. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.R.; Frevert, C.W. Innate Immunity in the Lungs. Proc. Am. Thorac. Soc. 2005, 2, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Sidletskaya, K.; Vitkina, T.; Denisenko, Y. The Role of Toll-Like Receptors 2 and 4 in the Pathogenesis of Chronic Obstructive Pulmonary Disease. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 1481–1493. [Google Scholar] [CrossRef]
- Uemasu, K.; Tanabe, N.; Tanimura, K.; Hasegawa, K.; Mizutani, T.; Hamakawa, Y.; Sato, S.; Ogawa, E.; Thomas, M.J.; Ikegami, M.; et al. Serine Protease Imbalance in the Small Airways and Development of Centrilobular Emphysema in COPD. Am. J. Respir. Cell Mol. Biol. 2020, 63, 67–78. [Google Scholar] [CrossRef]
- Kurotani, R.; Ono, S.; Miyano, Y.; Nakayama, S.; Liu, H.; Aibara, D.; Sakahara, S.; Sato, M.; Sato, K.; Inoue, S.; et al. Secretoglobin 3A2 Protects Lung from Developing Cigarette Smoke-Induced Pulmonary Emphysema. Int. J. Biochem. Cell Biol. 2023, 157, 106390. [Google Scholar] [CrossRef]
- Quinn, M.; Turner, A.M. Modernising Case Finding for α1-Antitrypsin Deficiency by DNA Sequencing of COPD Patients. Eur. Respir. J. 2020, 56, 2002628. [Google Scholar] [CrossRef]
- Agarwal, A.; Rizwana; Tripathi, A.D.; Kumar, T.; Sharma, K.P.; Patel, S.K.S. Nutritional and Functional New Perspectives and Potential Health Benefits of Quinoa and Chia Seeds. Antioxidants 2023, 12, 1413. [Google Scholar] [CrossRef]
- Miklós, Z.; Horváth, I. The Role of Oxidative Stress and Antioxidants in Cardiovascular Comorbidities in COPD. Antioxidants 2023, 12, 1196. [Google Scholar] [CrossRef]
- Audousset, C.; McGovern, T.; Martin, J.G. Role of Nrf2 in Disease: Novel Molecular Mechanisms and Therapeutic Approaches—Pulmonary Disease/Asthma. Front. Physiol. 2021, 12, 727806. [Google Scholar] [CrossRef]
- Pasini, A.M.F.; Stranieri, C.; Ferrari, M.; Garbin, U.; Cazzoletti, L.; Mozzini, C.; Spelta, F.; Peserico, D.; Cominacini, L. Oxidative Stress and Nrf2 Expression in Peripheral Blood Mononuclear Cells Derived from COPD Patients: An Observational Longitudinal Study. Resp. Respir. 2020, 21, 37. [Google Scholar] [CrossRef] [PubMed]
- Guan, P.; Cai, W.; Zhong, C.; Jiang, F.; Wu, J.; Zhai, X. A Low Expression of NRF2 Enhances Oxidative Stress and Autophagy in Myofibroblasts, Promoting Progression of Chronic Obstructive Pulmonary Disease. Curr. Med. Chem. 2024. [Google Scholar] [CrossRef] [PubMed]
- Sotgia, S.; Paliogiannis, P.; Sotgiu, E.; Mellino, S.; Zinellu, E.; Fois, A.G.; Pirina, P.; Carru, C.; Mangoni, A.A.; Zinellu, A. Systematic Review and Meta-Analysis of the Blood Glutathione Redox State in Chronic Obstructive Pulmonary Disease. Antioxidants 2020, 9, 1146. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, R.; Wei, X.; Zhang, C.; Pei, W.; Zhang, X.; Yang, Z.; Li, Z.; Zhang, Y.; Shi, Y.; et al. GSTP1 rs4147581 C>G and NLRP3 rs3806265 T>C as Risk Factors for Chronic Obstructive Pulmonary Disease: A Case-Control Study. Int. J. Chronic Obstr. Pulm. Dis. 2024, 19, 489–500. [Google Scholar] [CrossRef]
- Chen, M.; Xu, K.; He, Y.; Jin, J.; Mao, R.; Gao, L.; Zhang, Y.; Wang, G.; Gao, P.; Xie, M.; et al. CC16 as an Inflammatory Biomarker in Induced Sputum Reflects Chronic Obstructive Pulmonary Disease (COPD) Severity. Int. J. Chronic Obstr. Pulm. Dis. 2023, 18, 705–717. [Google Scholar] [CrossRef]
- Ellingsen, J.; Janson, C.; Bröms, K.; Hårdstedt, M.; Högman, M.; Lisspers, K.; Palm, A.; Stallberg, B.; Malinovschi, A. CRP, Fibrinogen, White Blood Cells, and Blood Cell Indices as Prognostic Biomarkers of Future COPD Exacerbation Frequency: The TIE Cohort Study. J. Clin. Med. 2024, 13, 3855. [Google Scholar] [CrossRef]
- Qin, L.; Guitart, M.; Curull, V.; Sánchez-Font, A.; Duran, X.; Tang, J.; Admetllo, M.; Barreiro, E. Systemic Profiles of microRNAs, Redox Balance, and Inflammation in Lung Cancer Patients: Influence of COPD. Biomedicines 2021, 9, 1347. [Google Scholar] [CrossRef]
- Ditz, B.; Kistemaker, L.E.M.; Van Den Berge, M.; Vonk, J.M.; Gosens, R.; Kerstjens, H.A.M. Responsivity and Reproducibility of Sputum Inflammatory Biomarkers During COPD Exacerbation and Stable Phases—A Pilot Study. Int. J. Chronic Obstr. Pulm. Dis. 2021, 16, 3055–3064. [Google Scholar] [CrossRef]
| Potential Factors | Characteristics/Agent | Changes in Lungs/Cells | Molecular Markers/Analysis | References |
|---|---|---|---|---|
| Cigarette smoking | Programmed cell death and inflammation in lung cells | Apoptosis, immune response, cell adhesion, and inflammation | A whole-genome transcriptomics analysis of blood monocytes (principal component analysis and hierarchical component analysis performed for genes CASP9 and TNFRSF1A) | [34] |
| Chronic bronchitis | Inflammation and lung injury. Toxic and mutagenic chemicals that impair regulatory framework of the cells | COPD diagnosis relies on the FEV1/FVC ratio post-bronchodilator set by the Global Lung Function Initiative | [35] | |
| Genetic | Lung function impairment in COPD patients | Terminal respiratory insufficiency and lung injury | Alpha-1 antitrypsin deficiency (imbalances in protease–antiprotease protection in the lung) | [41,42] |
| Accelerated aging | Cellular senescence in lung cells | Increase in alveolar space and a decrease in elastic rebound | The effect was assessed by examining DNA double-strand breaks and senescence levels in BOEC from smokers and COPD patients compared to healthy nonsmokers. Changes in the Sirtuin 1 (SIRT1) expression | [48] |
| Aged and young pulmonary cells exposure to cigarette smoke | Increased expression of age-related genes, regulating apoptosis, cytokine expression, and antioxidant activity | Aging-related genes in COPD were investigated using bioinformatic analyses, identifying 24 candidate genes enriched in cytokine activity, apoptosis, NF-κB, and IL-17 signaling, with four genes (CDKN1A, HIF1A, MXD1, and SOD2) | [49] | |
| Air pollution | Long-term ozone exposure, and PM2.5 exposure | Pollutants cause lung function to deteriorate and aggravate the COPD symptoms | Pollutant-induced inflammatory cytokine expression | [59,60,61] |
| Respiratory infection | Haemophilus influenzae | Bronchial infections elevated sputum IL-1β and tumor necrosis factor α | 1706 sputum samples from 510 COPD patients were integratively analyzed, using COPDMAP/AERIS as discovery sets and BEAT-COPD for validation. | [67] |
| Pseudomonas aeruginosa | Biofilm formation in COPD lungs | Analysis of sputum samples and recommendations for antibiotic treatment in stable COPD with chronic bronchial infection | [66] | |
| Aspergillus sp. | Lung cell infection | Overview of clinical Aspergillus signatures in COPD and bronchiectasis, covering advances in understanding the mycobiome using next-generation sequencing | [70] | |
| COVID-19 | Impaired lung functions | Evidence indicates that COVID-19 outcomes are exacerbated in COPD patients | [71] |
| Immune Cells | Function/Role | Effect | Molecular Signatures | References |
|---|---|---|---|---|
| Neutrophils (Section 3.1) | Protease-induced tissue damage | Acute exacerbations | IL-8 and CXC chemokines in COPD airways | [78,81] |
| Macrophages (Section 3.2) | Regulation of inflammation, tissue remodeling, and impaired phagocytosis | Sustained inflammation, emphysema, and impaired clearance of pathogens | TNF-α, IL-1β, IL-6, IL-8, ROS, MMPs (e.g., MMP-9), NF-κB, and STATs | [85,86,87] |
| Monocytes (Section 3.3) | Phagocytosis, cell migration, cytokine secretion, apoptosis, membrane vesicle shedding and differentiation | Alveolar epithelial cell death-driven lung tissue damage | Monocyte chemotactic protein-1. | [88,89,93] |
| Eosinophils (Section 3.4) | Inflammation, cell migration, release of cellular mediators, antimicrobial activity, and allergic reactions | Increase in alveolar space and a decrease in elastic rebound. Increase expression of age-related genes that regulates apoptosis, cytokine expression and antioxidant activity | Pro-eosinophilic mediators | [94,98] |
| T-lymphocytes (Section 3.5) | Pathogen infection in COPD patients | Development of lymphoid follicles | CD4+, CD8+, and IL-17 | [100,102] |
| B-lymphocytes (Section 3.6) | Extracellular remodeling | Fibrosis/collagen deposition in lungs tissue | CD27⁺ expressed differential profiles of CCR7, CCR6, CXCR4, and CXCR receptors | [108] |
| Inflammatory Markers | Activation Factor | Observations | References |
|---|---|---|---|
| Cytokines (Section 4.1) | Smoking cigarettes and aging | TNF-α-mediated inflammation | [128] |
| Inflammation or tissue damage | IL-1 cytokine expression | [129] | |
| Cigarette smoke (CS) and bacterial lipopolysaccharide-induced inflammation | Activation of SOCS3 and TRAF1 signaling | [131] | |
| MicroRNA-126 (miR-126) release | Inflammatory cytokines release | [132] | |
| C-reactive protein (CRP) and serum albumin (Section 4.2) | CRP/serum albumin ratio as potential biomarker | Inflammation-mediated CRP and albumin concentrations in the blood | [139] |
| Fibrinogen (Section 4.3) | Inflammation, fibrosis, and tissue injury | Upregulate the synthesis of acute-phase fibrinogens in the blood | [142,144] |
| sRAGE (Section 4.4) | Lower levels of sRAGE linked with exacerbating pulmonary function | Prevents the downstream inflammatory signaling | [146,147] |
| CC16 (Section 4.5) | CC16 exhibits anti-inflammatory properties | CC16 expression is induced by ozone, allergens, and viruses | [148,149] |
| Surfactant protein D (Section 4.6) | Lipid hemostasis and regulation of surfactants | High level of SP-D in serum | [151] |
| Adiponectin (Section 4.7) | Biomarker for the severity and development of COPD | High expression of APN | [155] |
| ROS (Section 4.8) | Tissue injury results in increased oxidative stress | Regulation of inflammatory transcription pathways, inhibition of sirtunin-1, and induces DNA damage | [158,159] |
| Proteases (Section 4.9) | Emphysema, neutrophil-mediated inflammation, and arterial stiffness | Elastases and MMPs expression in lungs | [161] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pankush; Bharti, K.; Pandey, R.; Srivastava, N.; Kashyap, S.; Kumar, D.; Kumar, L.; Suman, S.K.; Patel, S.K.S. Role of Inflammatory Mediators in Chronic Obstructive Pulmonary Disease Pathogenesis: Updates and Perspectives. Immuno 2025, 5, 13. https://doi.org/10.3390/immuno5020013
Pankush, Bharti K, Pandey R, Srivastava N, Kashyap S, Kumar D, Kumar L, Suman SK, Patel SKS. Role of Inflammatory Mediators in Chronic Obstructive Pulmonary Disease Pathogenesis: Updates and Perspectives. Immuno. 2025; 5(2):13. https://doi.org/10.3390/immuno5020013
Chicago/Turabian StylePankush, Khushboo Bharti, Rohit Pandey, Namita Srivastava, Shashank Kashyap, Deepak Kumar, Lokender Kumar, Sunil K. Suman, and Sanjay K. S. Patel. 2025. "Role of Inflammatory Mediators in Chronic Obstructive Pulmonary Disease Pathogenesis: Updates and Perspectives" Immuno 5, no. 2: 13. https://doi.org/10.3390/immuno5020013
APA StylePankush, Bharti, K., Pandey, R., Srivastava, N., Kashyap, S., Kumar, D., Kumar, L., Suman, S. K., & Patel, S. K. S. (2025). Role of Inflammatory Mediators in Chronic Obstructive Pulmonary Disease Pathogenesis: Updates and Perspectives. Immuno, 5(2), 13. https://doi.org/10.3390/immuno5020013







