Preliminary Evidence of Enhanced Immunogenicity of Hepatitis B Virus Vaccines When Co-Administered with Calcium Phosphate, Aluminum Hydroxide, and Cytosine Phospho-Guanine Oligodeoxynucleotides Combined Adjuvant in BALB/c Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Approval
2.2. Animal Model
2.3. Study Design and Sample Size Determination
2.4. Adjuvant Combination and Immunization Protocol
2.5. Samples Collection
2.6. Assessment of Humoral Immune Response
2.7. Extraction of Total RNA from Spleen Tissue
2.8. cDNA Synthesis and Relative Quantification of IL-6 and TNF Alpha mRNA Levels
2.9. Assessment of Hematological and Biochemical Analysis
2.10. Data Analysis
3. Results
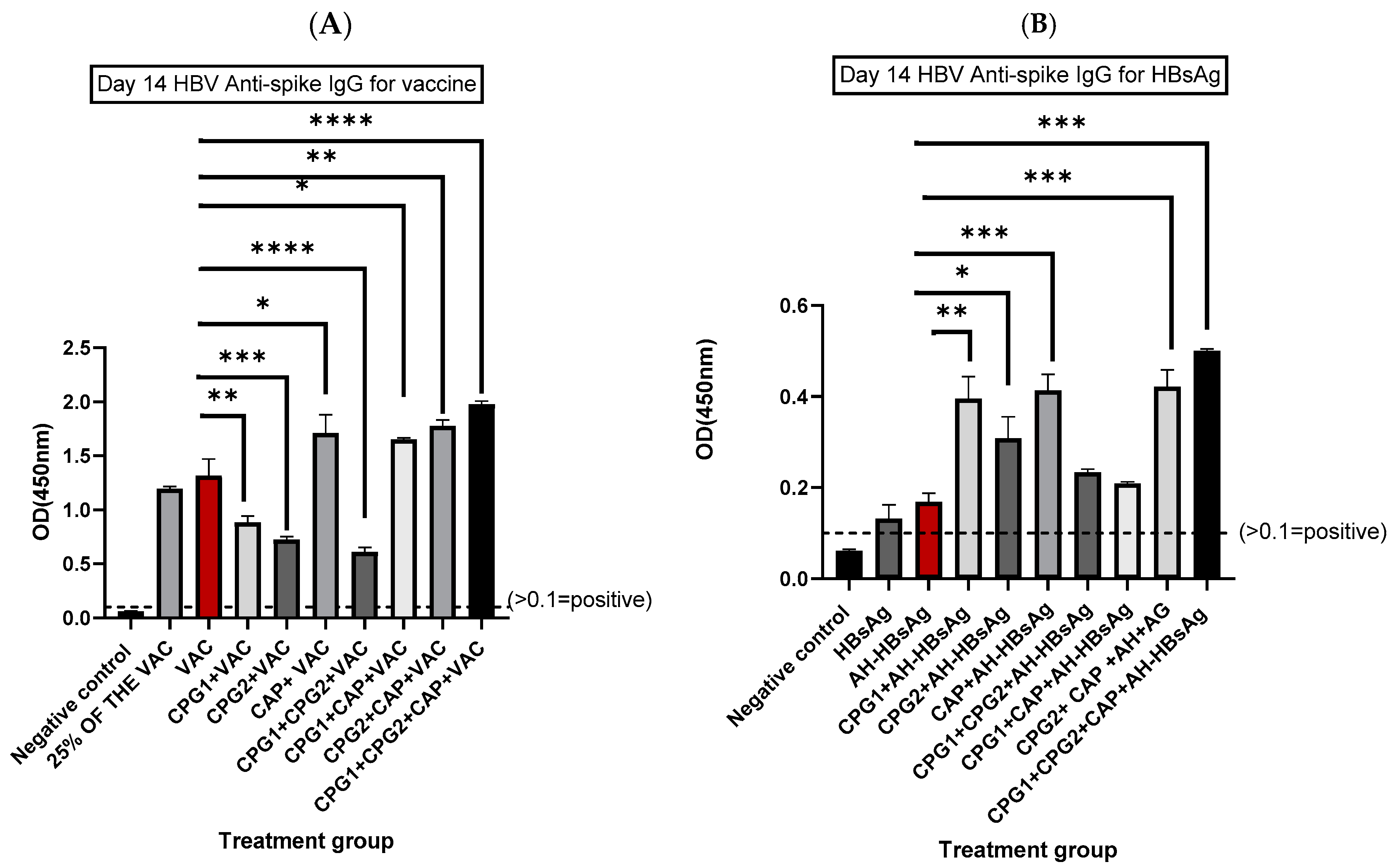
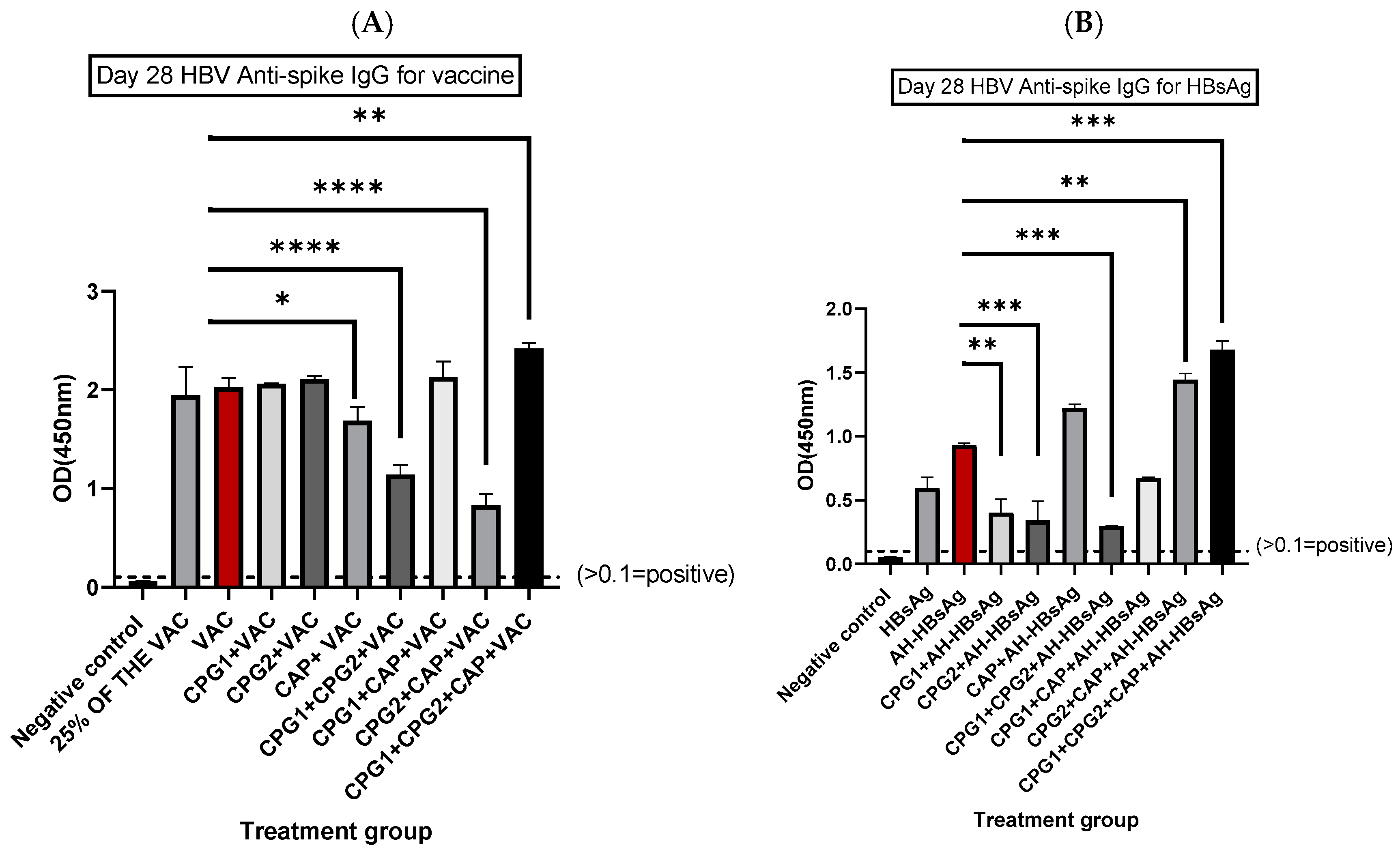
3.1. Determination of HBV Spike-Specific IgG Antibodies at Days 14 and 28 Post-Immunization
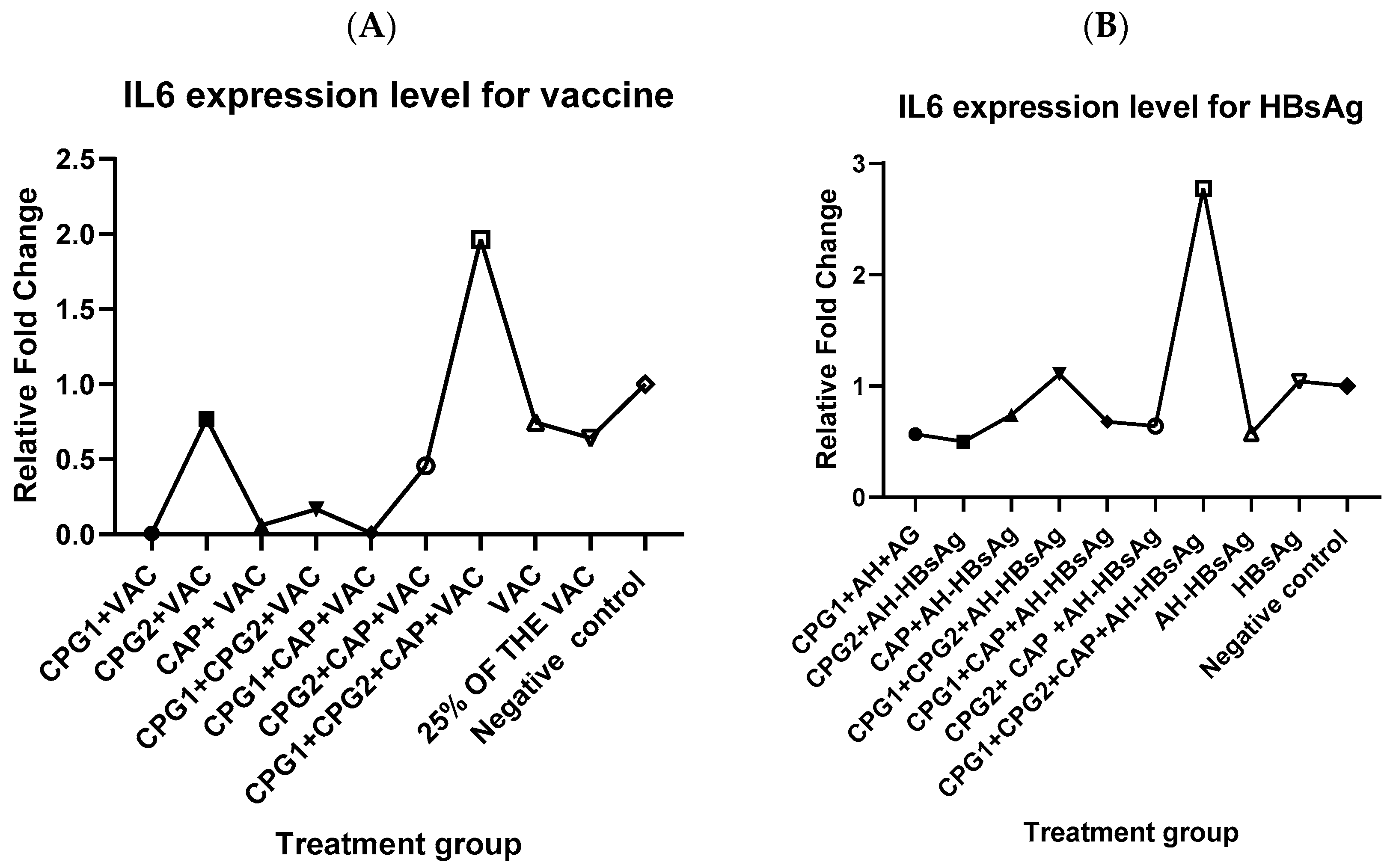
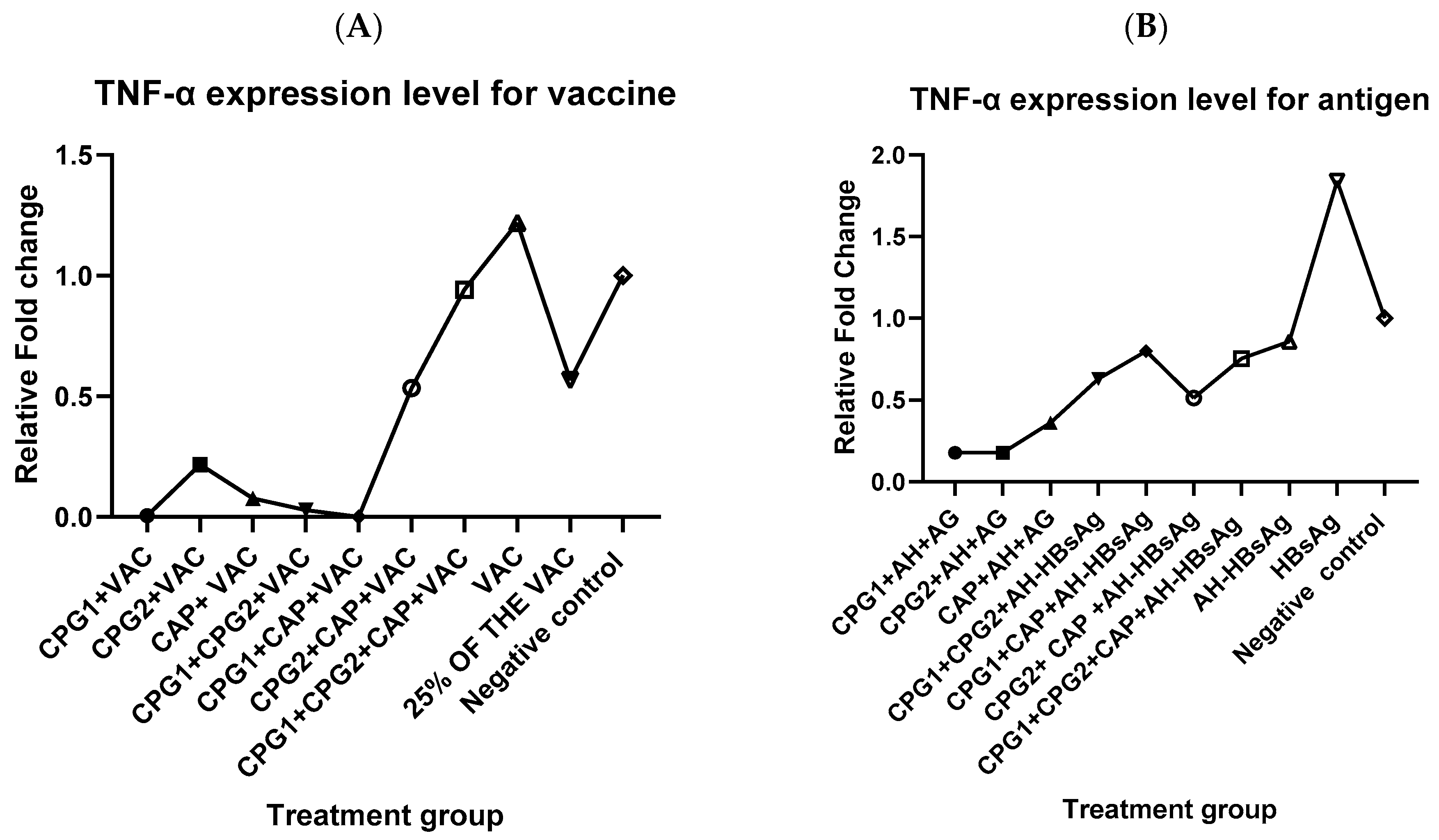
3.2. Effect of Most Potent Adjuvant Combination on IL-6 and TNF-α Expression
3.3. Assessment of Hematological and Biochemical Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leroux-Roels, G. Old and new adjuvants for hepatitis B vaccines. Med. Microbiol. Immunol. 2015, 204, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Qiu, J.; Xiang, Q.; Yi, J.; Zhu, J.; Zhao, Q. Epidemiology of hepatitis B virus infection among preconception couples in South China: A cross-sectional study. BMJ Open 2023, 13, e061165. [Google Scholar] [CrossRef]
- Yuen, M.-F.; Chen, D.-S.; Dusheiko, G.M.; Janssen, H.L.A.; Lau, D.T.Y.; Locarnini, S.A.; Peters, M.G.; Lai, C.-L. Hepatitis B virus infection. Nat. Rev. Dis. Prim. 2018, 4, 18036. [Google Scholar] [CrossRef] [PubMed]
- Bishop, N. Hepatitis B. Contin. Pract. 1992, 19, 35–38. [Google Scholar]
- Meireles, L.C. Three decades of hepatitis B control with vaccination. World J. Hepatol. 2015, 7, 2127–2132. [Google Scholar] [CrossRef]
- Ndung’U, F.; Nyanjom, S.; Omari, S.; Wainaina, E.; Mugasiali, R.; Kimotho, J. Preliminary assessment of adjuvant activities of Glycine max (L.) Merr saponin extract in BALB/c mice immunized with hepatitis B virus vaccine. F1000Research 2023, 12, 1145. [Google Scholar] [CrossRef]
- Di Lello, F.A.; Martínez, A.P.; Flichman, D.M. Insights into induction of the immune response by the hepatitis B vaccine. World J. Gastroenterol. 2022, 28, 4249–4262. [Google Scholar] [CrossRef]
- Mount, A.; Koernig, S.; Silva, A.; Drane, D.; Maraskovsky, E.; Morelli, A.B. Combination of adjuvants: The future of vaccine design. Expert Rev. Vaccines 2013, 12, 733–746. [Google Scholar] [CrossRef]
- Coffman, R.L.; Sher, A.; Seder, R.A. Vaccine adjuvants: Putting innate immunity to work. Immunity 2010, 33, 492–503. [Google Scholar] [CrossRef]
- Jangra, S.; Landers, J.J.; Rathnasinghe, R.; O’konek, J.J.; Janczak, K.W.; Cascalho, M.; Kennedy, A.A.; Tai, A.W.; Baker, J.R.; Schotsaert, M.; et al. A Combination Adjuvant for the Induction of Potent Antiviral Immune Responses for a Recombinant SARS-CoV-2 Protein Vaccine. Front. Immunol. 2021, 12, 729189. [Google Scholar] [CrossRef]
- Levast, B.; Awate, S.; Babiuk, L.; Mutwiri, G.; Gerdts, V.; Hurk, S.V.D.L.-V.D. Vaccine potentiation by combination adjuvants. Vaccines 2014, 2, 297–322. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Kaur, H.; Kaur, S. Evaluation of the immunoprophylactic potential of a killed vaccine candidate in combination with different adjuvants against murine visceral leishmaniasis. Parasitol. Int. 2015, 64, 70–78. [Google Scholar] [CrossRef]
- Mutwiri, G.; Gerdts, V.; Hurk, S.v.D.L.-V.D.; Auray, G.; Eng, N.; Garlapati, S.; Babiuk, L.A.; Potter, A. Combination adjuvants: The next generation of adjuvants? Expert Rev. Vaccines 2011, 10, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Egelston, C.; Gagnon, S.; Sui, Y.; Belyakov, I.M.; Klinman, D.M.; Berzofsky, J.A. Using 3 TLR ligands as a combination adjuvant induces qualitative changes in T cell responses needed for antiviral protection in mice. J. Clin. Investig. 2010, 120, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Shirota, H.; Klinman, D. CpG Oligodeoxynucleotides as Adjuvants for Clinical Use. In Immunopotentiators in Modern Vaccines; Elsevier: Amsterdam, The Netherlands, 2017; pp. 163–198. [Google Scholar]
- Celise, D.A.; Kimotho, J.; Kimani, J.W.; Muriithi, A.K.; Odari, E.O. Increase in the Immune Response in Balb/c Mice after the Co-Administration of a Vector-Based COVID-19 Vaccine with Cytosine Phosphoguanine Oligodeoxynucleotide. Vaccines 2023, 11, 53. [Google Scholar] [CrossRef]
- Kemi, T.; Nelly, S.; Kariuki, D.; Kimotho, J. Co-administration of a Hepatitis B vaccine with CpG-ODN 2395 induces stronger immune response in BALB/c mice. F1000Research 2024, 13, 404. [Google Scholar] [CrossRef]
- Masson, J.-D.; Thibaudon, M.; Bélec, L.; Crépeaux, G. Calcium phosphate: A substitute for aluminum adjuvants? Expert Rev. Vaccines 2017, 16, 289–299. [Google Scholar] [CrossRef]
- Moni, S.S.; Abdelwahab, S.I.; Jabeen, A.; Elmobark, M.E.; Aqaili, D.; Gohal, G.; Oraibi, B.; Farasani, A.M.; Jerah, A.A.; Alnajai, M.M.A.; et al. Advancements in Vaccine Adjuvants: The Journey from Alum to Nano Formulations. Vaccines 2023, 11, 1704. [Google Scholar] [CrossRef]
- Math: Permutation and Combination. 2010. Available online: https://sathee.prutor.ai/article/maths/permutation-and-combination/# (accessed on 11 August 2024).
- Atmaca, H.T. Expression of serotonin 2A, 2C, 6 and 7 receptor and IL-6 mRNA in experimental toxoplasmic encephalitis in mice. Heliyon 2019, 5, e02890. [Google Scholar] [CrossRef]
- Sudo, K.; Yamada, Y.; Saito, K.; Shimizu, S.; Ohashi, H.; Kato, T.; Moriwaki, H.; Ito, H.; Seishima, M. TNF-α and IL-6 signals from the bone marrow derived cells are necessary for normal murine liver regeneration. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2008, 1782, 671–679. [Google Scholar] [CrossRef]
- Medrano, G.; Guan, P.; Barlow-Anacker, A.J.; Gosain, A. Comprehensive selection of reference genes for quantitative RT-PCR analysis of murine extramedullary hematopoiesis during development. PLoS ONE 2017, 12, e0181881. [Google Scholar] [CrossRef] [PubMed]
- Kindrachuk, J.; Jenssen, H.; Elliott, M.; Townsend, R.; Nijnik, A.; Lee, S.F.; Gerdts, V.; Babiuk, L.A.; Halperin, S.A.; Hancock, R.E. A novel vaccine adjuvant comprised of a synthetic innate defence regulator peptide and CpG oligonucleotide links innate and adaptive immunity. Vaccine 2009, 27, 4662–4671. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Li, W.; Lenzo, J.C.; Holden, J.A.; McCullough, M.J.; O’connor, A.J.; O’brien-Simpson, N.M. The Potential of Calcium Phosphate Nanoparticles as Adjuvants and Vaccine Delivery Vehicles. Front. Mater. 2021, 8, 788373. [Google Scholar] [CrossRef]
- Le, C.T.T.; Ahn, S.Y.; Ho, T.L.; Lee, J.; Lee, D.-H.; Hwang, H.S.; Kang, S.-M.; Ko, E.-J. Adjuvant effects of combination monophosphoryl lipid A and poly I:C on antigen-specific immune responses and protective efficacy of influenza vaccines. Sci. Rep. 2023, 13, 12231. [Google Scholar] [CrossRef]
- Haseda, Y.; Munakata, L.; Kimura, C.; Kinugasa-Katayama, Y.; Mori, Y.; Suzuki, R.; Aoshi, T. Development of combination adjuvant for efficient T cell and antibody response induction against protein antigen. PLoS ONE 2021, 16, e0254628. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Liu, S.; Liu, S.; Li, B.; Li, B.; Sun, X.; Sun, X.; Pan, Q.; Pan, Q.; et al. A novel CpG ODN compound adjuvant enhances immune response to spike subunit vaccines of porcine epidemic diarrhea virus. Front. Immunol. 2024, 15, 1336239. [Google Scholar] [CrossRef]
- Ebensen, T.; Delandre, S.; Prochnow, B.; Guzmán, C.A.; Schulze, K. The Combination Vaccine Adjuvant System Alum/c-di-AMP Results in Quantitative and Qualitative Enhanced Immune Responses Post Immunization. Front. Cell. Infect. Microbiol. 2019, 9, 31. [Google Scholar] [CrossRef]
- Sichangi, M.W. Understanding Mechanisms of Actions for Vaccine Adjuvants Critical for Designing Effective Vaccines: A narrative review. Afr. J. Health Sci. 2020, 33, 18–29. Available online: https://www.ajol.info/index.php/ajhs/article/view/202031 (accessed on 25 February 2025).
- Lan, T.; Chang, L.; Wu, L.; Yuan, Y.-F. Il-6 plays a crucial role in hbv infection. J. Clin. Transl. Hepatol. 2015, 3, 271–276. [Google Scholar] [CrossRef]
- Kuo, T.-M.; Hu, C.-P.; Chen, Y.-L.; Hong, M.-H.; Jeng, K.-S.; Liang, C.-C.T.; Chen, M.-L.; Chang, C. HBV replication is significantly reduced by IL-6. J. Biomed. Sci. 2009, 16, 41. [Google Scholar] [CrossRef]
- Karaba, A.H.; Zhu, X.M.; Benner, S.E.; Akinde, O.B.; Eby, Y.; Wang, K.H.B.; Saraf, S.B.; Garonzik-Wang, J.M.; Klein, S.L.; Bailey, J.R.; et al. Higher Proinflammatory Cytokines Are Associated With Increased Antibody Titer After a Third Dose of SARS-CoV-2 Vaccine in Solid Organ Transplant Recipients. Transplantation 2022, 106, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Idriss, H.T.; Naismith, J.H. TNFα and the TNF receptor superfamily: Structure-function relationship(s). Microsc. Res. Tech. 2000, 50, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Silva-Santana, G.; Bax, J.C.; Fernandes, D.C.S.; Bacellar, D.T.L.; Hooper, C.; Dias, A.A.S.O.; Silva, C.B.; de Souza, A.M.; Ramos, S.; Santos, R.A.; et al. Clinical hematological and biochemical parameters in Swiss, BALB/c, C57BL/6 and B6D2F1 Mus musculus. Anim. Model. Exp. Med. 2020, 3, 304–315. [Google Scholar] [CrossRef]
- Jiang, D.; Portuguese, A.J.; Weatherford, A.; Garcia, D.; Gernsheimer, T. Platelet trends after COVID-19 vaccination in patients with chronic or persistent immune thrombocytopenia. Am. J. Hematol. 2021, 96, E472–E474. [Google Scholar] [CrossRef] [PubMed]
- Capllonch, A.; de Pablo, S.; de la Torre, A.; Morales, I. Increase in white cell and neutrophil counts during the first eighteen weeks of treatment with clozapine in patients admitted to a long-term psychiatric care inpatient unit. Span. J. Psychiatry Ment. Health 2018, 11, 94–100. [Google Scholar] [CrossRef]
- Imamura, M.; Morimoto, T.; Egawa, C.; Fukui, R.; Bun, A.; Ozawa, H.; Miyagawa, Y.; Fujimoto, Y.; Higuchi, T.; Miyoshi, Y. Significance of baseline neutrophil-to-lymphocyte ratio for progression-free survival of patients with HER2-positive breast cancer treated with trastuzumab emtansine. Sci. Rep. 2019, 9, 1811. [Google Scholar] [CrossRef]


| Adjuvant Combination | Administered Dosage |
|---|---|
| CpG-ODN 2395 [CpG1] | 0.67 μM of CpG-ODN 2395 + 1 μg (50 μL) of BEVAC Vaccine |
| 0.67 μM of CpG-ODN 2395 + 1.2 mg of AH + 0.01 μg (50 μL) of HBs Antigen | |
| CpG-ODN18281-2 [CpG2] | 0.67 μM of CpG-ODN18281-2 + 1 μg (50 μL) of BEVAC Vaccine |
| 0.67 μM of CpG-ODN18281-2 +1.2 mg of AH +0.01 μg (50 μL) of HBs Antigen | |
| CAP | 1.3 mg of CAP + 1 μg(50 μL) of BEVAC Vaccine |
| 1.3 mg of CAP + 1.2 mg of AH + 0.01 μg(50 μL) of HBs Antigen | |
| CpG-ODN 2395 [CpG1] +CpG-ODN18281-2 [CpG2] | 0.67 μM of CpG-ODN 2395 + 0.67 μg of CpG-ODN18281-2 + 1 μg (50 μL) of BEVAC Vaccine |
| 0.67 μM of CpG-ODN 2395 + 0.67 μg of CpG-ODN18281-2 + 1.2 mg of AH + 0.01 μg (50 uL) of HBs Antigen | |
| CpG-ODN 2395 [CpG1] + CAP | 0.67 μg of CpG-ODN 2395 + 1.3 mg of CAP + 1 μg(50 μL) of BEVAC Vaccine |
| 0.67 μg of CpG-ODN 2395 + 1.3 mg of CAP +1.2 mg of AH + 0.01 μg (50 μL) of HBs Antigen | |
| CpG-ODN18281-2 [CpG2] +CAP | 0.67 μM of CpG-ODN18281-2 + 1.3 mg of CAP +1 μg(50 μL) of BEVAC Vaccine |
| 0.67 μM of CpG-ODN18281-2 +1.3 mg of CAP +1.2 mg of AH +0.01 μg (50 μL) of HBs Antigen | |
| CpG-ODN 2395 [CpG1] +CpG-ODN18281-2 [CpG2] +CAP | 0.67 μM of CpG-ODN 2395 + 0.67μM of CpG-ODN18281-2 + 1.3 mg of CAP + 1 μg (50 μL) of BEVAC Vaccine |
| 0.67 μM of CpG-ODN 2395 + 0.67μM of CpG-ODN18281-2 + 1.3 mg of CAP +1.2 mg of AH + 0.01 μg (50 μL) of HBs Antigen | |
| Vaccine Alone | 1 μg (50 μL) of the BEVAC vaccine |
| 25% of Vaccine | 0.25 μg of BEVAC vaccine + 37.5 μL of PBS |
| Aluminum Hydroxide (AH) + Antigen (AG) | 1.2 mg of AH + 0.01 μg (50 μL) of HBs Antigen |
| Antigen Alone | 0.01 μg (50 μL) of HBs Antigen |
| Negative Control | 50 μL of PBS |
| Cytokine | Forward Primer | Reverse Primer | NCBI | Size of Amplicon | Reference |
|---|---|---|---|---|---|
| Interleukin 6 | 5′GAGGATACCACTCCCAACAGACC′3 | 5′AAGTGCATCATCGTTGTTCATACA3′ | XM_021163844.1 | 141 bp | [21] |
| Tumor Necrosis Factor (TNF) | 5′GATCTCAAAGACAACCAACATGTG 3′ | 5′CTCCAGCTGGAAGACTCCTCCCAG 3′ | NM_013693.3 | 76 bp | [22] |
| HPRT1 (Hypoxanthine guanine phosphoribosyl transferase 1) | 5′TCCTCCTCAGACCGCTTTT3′ | 5′CCTGGTTCATCATCGCTAATC3′ | NM_013556.2 90 | 90 bp | [23] |
| Parameter | CPG1 + CPG2 + CAP + VAC | CPG1 + CPG2 + CAP + AH-HBsAg | VAC | AH-HBsAg | Negative Control (PBS) | Range for Mice |
|---|---|---|---|---|---|---|
| WBC (103/UL) | 1.5 ± 0.4 | 1.45 ± 0.07 | 3.71 ± 2.7 | 1.55 ± 0.3 | 1.4 ± 0.4 | 1.09–11.3 |
| Neutrophils (%) | 38.5± 7.7 | 35 ± 7.0 | 31.5 ± 2.1 | 39 ± 8.4 | 59 ± 21.2 | 11–29 |
| Lymphocytes (%) | 46 ± 8.4 | 52 ± 5.6 | 54.5 ± 3.53 | 43 ± 7.7 | 24.5 ± 0.7 | 65–87 |
| Monocytes (%) | 14.5 ± 0.7 | 12 ± 1.4 | 12.5 ± 0.7 | 16.5 ± 0.7 | 15.5 ± 0 | 3.75–14.3 |
| Eosinophils (%) | 1 ± 0 | 1 ± 0 | 1.5 ± 0.7 | 1 ± 0 | 1 ± 0 | 0–5 |
| Basophils (%) | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0–1 |
| RBC (106/UL) | 8.235 ± 1.2 | 8.5 ± 0.2 | 8.32 ± 1.3 | 9.1± 0.2 | 9.025 ± 0.9 | 7.8–10.3 |
| HB (g/dl) | 10.4 ± 1.9 | 10.85 ± 2.3 | 9.75 ± 1 | 10.1 ± 3.1 | 11.1 ± 1.5 | 11.6–15.9 |
| HCT (%) | 34.85 ± 5.58 | 33 ± 3.8 | 38.2 ± 10.3 | 31.1 ± 3.1 | 38.55 ± 7.1 | 35.2–49.9 |
| MCV (FI) | 42.2 ± 0.4 | 45.1 ± 3.5 | 45.4 ± 4.9 | 44.0 ± 4.3 | 42.5 ± 3.3 | 39.2–56.2 |
| MCH (Pg) | 12.55 ± 0.4 | 11.8 ± 0.4 | 11.75 ± 0.6 | 11.15 ± 0.9 | 12.2 ± 0.4 | 12.4–18.4 |
| MCHC | 29.75 ± 0.9 | 26.05 ± 4.3 | 28.55 ± 3.8 | 28.5 ± 5.5 | 28.85 ± 1.3 | 27.2–40.8 |
| Platelets | 438 ± 0.1 * | 571 ± 0.3 * | 514 ± 0.03 * | 494 ± 0.1 * | 759.5 ± 0.2 * | 322–798 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouattara, O.; Kimani, J.W.; Kimotho, J.H. Preliminary Evidence of Enhanced Immunogenicity of Hepatitis B Virus Vaccines When Co-Administered with Calcium Phosphate, Aluminum Hydroxide, and Cytosine Phospho-Guanine Oligodeoxynucleotides Combined Adjuvant in BALB/c Mice. Immuno 2025, 5, 12. https://doi.org/10.3390/immuno5010012
Ouattara O, Kimani JW, Kimotho JH. Preliminary Evidence of Enhanced Immunogenicity of Hepatitis B Virus Vaccines When Co-Administered with Calcium Phosphate, Aluminum Hydroxide, and Cytosine Phospho-Guanine Oligodeoxynucleotides Combined Adjuvant in BALB/c Mice. Immuno. 2025; 5(1):12. https://doi.org/10.3390/immuno5010012
Chicago/Turabian StyleOuattara, Oumou, Josephine W. Kimani, and James H. Kimotho. 2025. "Preliminary Evidence of Enhanced Immunogenicity of Hepatitis B Virus Vaccines When Co-Administered with Calcium Phosphate, Aluminum Hydroxide, and Cytosine Phospho-Guanine Oligodeoxynucleotides Combined Adjuvant in BALB/c Mice" Immuno 5, no. 1: 12. https://doi.org/10.3390/immuno5010012
APA StyleOuattara, O., Kimani, J. W., & Kimotho, J. H. (2025). Preliminary Evidence of Enhanced Immunogenicity of Hepatitis B Virus Vaccines When Co-Administered with Calcium Phosphate, Aluminum Hydroxide, and Cytosine Phospho-Guanine Oligodeoxynucleotides Combined Adjuvant in BALB/c Mice. Immuno, 5(1), 12. https://doi.org/10.3390/immuno5010012






