Effect of Mixing Technique on Physico-Chemical Characteristics of Blended Membranes for Gas Separation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of Membranes
2.2.2. Characterization of Membranes
Scanning Electron Microscopy
Thermal Analysis
FTIR Analysis
3. Results and Discussion
3.1. Morphological Analysis of Blended Membranes
3.2. FTIR Analysis of Blended Membranes
3.3. Thermogravimetric Analysis of Blended Membranes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kadirkhan, F.; Goh, P.S.; Ismail, A.F.; Wan Mustapa, W.N.F.; Halim, M.H.M.; Soh, W.K.; Yeo, S.Y. Recent advances of polymeric membranes in tackling plasticization and aging for practical industrial CO2/CH4 applications—A review. Membranes 2022, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Vatanpour, V.; Pasaoglu, M.E.; Barzegar, H.; Teber, O.O.; Kaya, R.; Bastug, M.; Khataee, A.; Koyuncu, I. Cellulose acetate in fabrication of polymeric membranes: A review. Chemosphere 2022, 295, 133914. [Google Scholar] [CrossRef] [PubMed]
- Mubashir, M.; Yeong, Y.F.; Lau, K.K.; Chew, T.L.; Norwahyu, J. Efficient CO2/N2 and CO2/CH4 separation using NH2-MIL-53 (Al)/cellulose acetate (CA) mixed matrix membranes. Sep. Purif. Technol. 2018, 199, 140–151. [Google Scholar] [CrossRef]
- Raza, A.; Farrukh, S.; Hussain, A.; Khan, I.; Othman, M.H.D.; Ahsan, M. Performance Analysis of Blended Membranes of Cellulose Acetate with Variable Degree of Acetylation for CO2/CH4 Separation. Membranes 2021, 11, 245. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Varghese, A.M.; Reddy, K.S.K.; Romanos, G.E.; Karanikolos, G.N. Polysulfone Mixed-Matrix Membranes Comprising Poly(ethylene glycol)-Grafted Carbon Nanotubes: Mechanical Properties and CO2 Separation Performance. Ind. Eng. Chem. Res. 2021, 60, 11289–11308. [Google Scholar] [CrossRef]
- Karimi, S.; Firouzfar, E.; Khoshchehreh, M.R. Assessment of gas separation properties and CO2 plasticization of polysulfone/polyethylene glycol membranes. J. Pet. Sci. Eng. 2019, 173, 13–19. [Google Scholar] [CrossRef]
- Douna, I.; Farrukh, S.; Hussain, A.; Salahuddin, Z.; Noor, T.; Pervaiz, E.; Younas, M.; Fan, X.F. Experimental investigation of polysulfone modified cellulose acetate membrane for CO2/H2 gas separation. Korean J. Chem. Eng. 2022, 39, 189–197. [Google Scholar] [CrossRef]
- Jami’an, W.N.R.; Hasbullah, H.; Mohamed, F.; Yusof, N.; Ibrahim, N.; Ali, R.R. Effect of evaporation time on cellulose acetate membrane for gas separation. IOP Conf. Ser. Earth Environ. Sci. 2016, 36, 012008. [Google Scholar] [CrossRef]
- Moghadassi, A.R.; Rajabi, Z.; Hosseini, S.M.; Mohammadi, M. Fabrication and modification of cellulose acetate based mixed matrix membrane: Gas separation and physical properties. J. Ind. Eng. Chem. 2014, 20, 1050–1060. [Google Scholar] [CrossRef]
- Kim, W.-g.; Lee, J.S.; Bucknall, D.G.; Koros, W.J.; Nair, S. Nanoporous layered silicate AMH-3/cellulose acetate nanocomposite membranes for gas separations. J. Membr. Sci. 2013, 441, 129–136. [Google Scholar] [CrossRef]
- Ali, M.; Zafar, M.; Jamil, T.; Butt, M.T.Z. Influence of glycol additives on the structure and performance of cellulose acetate/zinc oxide blend membranes. Desalination 2011, 270, 98–104. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Jawad, Z.A.; Low, S.C.; Zein, S.H.S. A cellulose acetate/multi-walled carbon nanotube mixed matrix membrane for CO2/N2 separation. J. Membr. Sci. 2014, 451, 55–66. [Google Scholar] [CrossRef]
- Lam, B.; Wei, M.; Zhu, L.; Luo, S.; Guo, R.; Morisato, A.; Alexandridis, P.; Lin, H. Cellulose triacetate doped with ionic liquids for membrane gas separation. Polymer 2016, 89, 1–11. [Google Scholar] [CrossRef]
- Miller-Chou, B.A.; Koenig, J.L. A review of polymer dissolution. Prog. Polym. Sci. 2003, 28, 1223–1270. [Google Scholar] [CrossRef]
- Kluge, S.; Kose, T.; Tutuş, M. Tuning the Morphology and Gas Separation Properties of Polysulfone Membranes. Membranes 2022, 12, 654. [Google Scholar] [CrossRef]
- Mubashir, M.; Fong, Y.; Leng, C.; Keong, L. Enhanced gases separation of cellulose acetate membrane using N-methyl-1-2 pyrrolidone as fabrication solvent. Int. J. Automot. Mech. Eng. 2018, 15, 4978–4986. [Google Scholar] [CrossRef]
- Li, Z.; Ren, J.; Fane, A.G.; Li, D.F.; Wong, F.-S. Influence of solvent on the structure and performance of cellulose acetate membranes. J. Membr. Sci. 2006, 279, 601–607. [Google Scholar] [CrossRef]
- Shieh, J.-J.; Chung, T.S. Effect of liquid-liquid demixing on the membrane morphology, gas permeation, thermal and mechanical properties of cellulose acetate hollow fibers. J. Membr. Sci. 1998, 140, 67–79. [Google Scholar] [CrossRef]
- Shiva Prasad, N.; Babarao, R.; Madapusi, S.; Sridhar, S.; Choudhury, N.R.; Bhargava, S.K. Residual solvent induced physical morphology and gas permeation in polyamide-imide membrane: Experimental investigation and molecular simulations. Eur. Polym. J. 2022, 165, 111012. [Google Scholar] [CrossRef]
- Ioniță, M.; Crică, L.E.; Voicu, S.I.; Dinescu, S.; Miculescu, F.; Costache, M.; Iovu, H. Synergistic effect of carbon nanotubes and graphene for high performance cellulose acetate membranes in biomedical applications. Carbohydr. Polym. 2018, 183, 50–61. [Google Scholar] [CrossRef]
- Dumitriu, C.; Voicu, S.I.; Muhulet, A.; Nechifor, G.; Popescu, S.; Ungureanu, C.; Carja, A.; Miculescu, F.; Trusca, R.; Pirvu, C. Production and characterization of cellulose acetate—Titanium dioxide nanotubes membrane fraxiparinized through polydopamine for clinical applications. Carbohydr. Polym. 2018, 181, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Corobea, M.C.; Muhulet, O.; Miculescu, F.; Antoniac, I.V.; Vuluga, Z.; Florea, D.; Vuluga, D.M.; Butnaru, M.; Ivanov, D.; Voicu, S.I. Novel nanocomposite membranes from cellulose acetate and clay-silica nanowires. Polym. Adv. Technol. 2016, 27, 1586–1595. [Google Scholar] [CrossRef]
- Badrinezhad, L.; Ghasemi, S.; Azizian-Kalandaragh, Y.; Nematollahzadeh, A. Preparation and characterization of polysulfone/graphene oxide nanocomposite membranes for the separation of methylene blue from water. Polym. Bull. 2018, 75, 469–484. [Google Scholar] [CrossRef]
- Saxena, M.; Sharma, S.; Bhattacharya, A. Recycling of polysulfone: Study properties of membranes. Int. J. Membr. Sci. Technol. 2015, 2, 39–46. [Google Scholar]
- Lee, T.H.; Lee, B.K.; Park, J.S.; Park, J.; Kang, J.H.; Yoo, S.Y.; Park, I.; Kim, Y.-H.; Park, H.B. Surface Modification of Matrimid®; 5218 Polyimide Membrane with Fluorine-Containing Diamines for Efficient Gas Separation. Membranes 2022, 12, 256. [Google Scholar] [CrossRef] [PubMed]
- Idris, A.; Man, Z.; Maulud, A.S.; Khan, M.S. Effects of phase separation behavior on morphology and performance of polycarbonate membranes. Membranes 2017, 7, 21. [Google Scholar] [CrossRef]



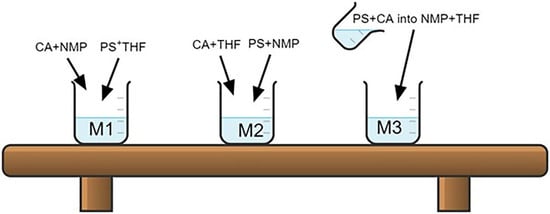
| Membrane Code | Polymer | Solvent | Mixing Method |
|---|---|---|---|
| M1 | CA (2.25 g) | NMP (11.25 mL) | MIX |
| PSU (0.25 g) | THF (11.25 mL) | MIX | |
| CA + PSU blend solutions together | |||
| M2 | CA (2.25 g) | THF (11.25 mL) | MIX |
| PSU (0.25 g) | NMP (11.25 mL) | MIX | |
| CA + PSU blend solutions together | |||
| M3 | By adding CA (2.25 g) + PSU (0.25 g) in a mixed solvent of NMP (11.25 mL) + THF (11.25 mL) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qadir, D.; Suleman, H.; Ahmad, F. Effect of Mixing Technique on Physico-Chemical Characteristics of Blended Membranes for Gas Separation. Gases 2023, 3, 136-143. https://doi.org/10.3390/gases3040009
Qadir D, Suleman H, Ahmad F. Effect of Mixing Technique on Physico-Chemical Characteristics of Blended Membranes for Gas Separation. Gases. 2023; 3(4):136-143. https://doi.org/10.3390/gases3040009
Chicago/Turabian StyleQadir, Danial, Humbul Suleman, and Faizan Ahmad. 2023. "Effect of Mixing Technique on Physico-Chemical Characteristics of Blended Membranes for Gas Separation" Gases 3, no. 4: 136-143. https://doi.org/10.3390/gases3040009
APA StyleQadir, D., Suleman, H., & Ahmad, F. (2023). Effect of Mixing Technique on Physico-Chemical Characteristics of Blended Membranes for Gas Separation. Gases, 3(4), 136-143. https://doi.org/10.3390/gases3040009